Key Points
Polyphosphate inhibitors are antithrombotics with a novel mechanism of action and reduced bleeding side effects compared with heparin.
Originally identified polyphosphate inhibitors were all toxic; this study reports the development of safe and effective alternatives.
Abstract
Polyphosphate (polyP) is secreted by activated platelets and has been shown to contribute to thrombosis, suggesting that it could be a novel antithrombotic target. Previously reported polyP inhibitors based on polycationic substances, such as polyethylenimine, polyamidoamine dendrimers, and polymyxin B, although they attenuate thrombosis, all have significant toxicity in vivo, likely due to the presence of multiple primary amines responsible for their polyP binding ability. In this study, we examined a novel class of nontoxic polycationic compounds initially designed as universal heparin reversal agents (UHRAs) to determine their ability to block polyP procoagulant activity and also to determine their utility as antithrombotic treatments. Several UHRA compounds strongly inhibited polyP procoagulant activity in vitro, and 4 were selected for further examination in mouse models of thrombosis and hemostasis. Compounds UHRA-9 and UHRA-10 significantly reduced arterial thrombosis in mice. In mouse tail bleeding tests, administration of UHRA-9 or UHRA-10 was associated with significantly less bleeding compared with therapeutically equivalent doses of heparin. Thus, these compounds offer a new platform for developing novel antithrombotic agents that target procoagulant anionic polymers such as polyP with reduced toxicity and bleeding side effects.
Introduction
Polyphosphate (polyP) is a highly anionic, linear polymer of inorganic phosphate that accumulates in many infectious microorganisms1 and is secreted by activated human platelets.2 Studies from our laboratory and others have shown that platelet polyP acts as a procoagulant stimulus at a number of points in the coagulation cascade.3,4 Although we do not currently understand all the mechanisms behind the ability of polyP to accelerate clotting, our present understanding of the role of platelet polyP in hemostasis and thrombosis suggests that it may contribute more heavily to thrombosis. Additionally, its role as an accelerant rather than a required component of the final common pathway of the coagulation cascade makes platelet polyP an attractive therapeutic target for novel antithrombotics with potentially decreased bleeding risk compared with conventional therapies, all of which target essential enzymes within the coagulation cascade.5
Cationic polymers make attractive candidates for high-affinity polyP inhibitors, and such polymers, including polyethylenimine and polyamidoamine (PAMAM) dendrimers, have proven effective in attenuating thrombosis in proof-of-principle studies that identified polyP as a therapeutic target.6,7 These polymers are positively charged because of the presence of multiple primary amines, which allows them to bind to and inhibit polyP, but this property can also promote binding to proteins and cell surfaces and thus lead to cellular toxicity, platelet activation, and coagulopathy mediated by fibrinogen aggregation.8,9 This severely limits the real-world usefulness of the previously identified polyP inhibitors.
Recently Kizhakkedathu and coworkers developed a family of dendritic polymer-based universal heparin reversal agents (UHRAs) as synthetic antidotes to all heparin-based anticoagulants.10 These UHRAs were designed by assembling multifunctional cationic groups into the core of a dendritic polymer; they are then shielded from nonspecific interactions with blood components by using a protective layer of short-chain polyethylene glycol (PEG), resulting in increased biocompatibility compared with conventional cationic polymers.
Although the development and synthesis of UHRA compounds resulted in the identification of important new heparin reversal agents, we also realized that within the UHRA family of compounds we might find polymer structures that could function as nontoxic polyP inhibitors. Their extremely low toxicity, coupled with the ease with which their chemical composition and pharmacologic properties can be varied, makes UHRA compounds ideal candidates for testing and developing this novel class of antithrombotic agents targeting polyP. This study reports the successful identification of UHRA compounds with high affinity for polyP in vitro that also interrupt thrombosis in vivo.
Methods
Synthesis of UHRAs
The polymer scaffolds of this family of UHRA compounds were synthesized by anionic ring-opening polymerization of glycidol and α-methoxy-ω-epoxy polyethylene glycol (mPEG-400), which were then postfunctionalized to introduce positively charged groups based on branched tertiary amines. Detailed synthetic methods are provided in the supplemental Methods, available on the Blood Web site.
UHRA biocompatibility studies
Blood from healthy consenting donors was collected by venipuncture under a protocol approved by the University of British Columbia clinical ethical committee, and written consent was obtained from each individual donor in accordance with the Declaration of Helsinki. Platelet-rich plasma (PRP) was prepared by centrifuging citrated whole blood samples at 150g for 10 minutes. Serum was prepared by centrifuging the blood collected in the serum tube at 1200g for 30 minutes, 1 hour after blood collection.
Platelet activation was quantified by flow cytometry. PRP was incubated at 37°C with UHRA, protamine, or polyethylenimine (PEI) (final concentration 1 or 2 mg/mL) in phosphate-buffered saline (PBS) for 1 hour. Five microliters of postincubation platelet-polymer mixture diluted in PBS was incubated for 20 minutes in the dark with 5 μL of phycoerythrin (PE) -labeled monoclonal anti-CD62P-PE (Immunotech). The samples were then stopped with 0.3 mL of PBS. The level of platelet activation was analyzed in a BD FACSCanto II flow cytometer (Becton Dickinson) and was expressed as a percentage of platelet activation marker CD62-PE fluorescence detected in 10 000 total events counted in gated platelets. PRP from 3 different donors was used for the analysis, and each sample was run in duplicate.
Complement activation was measured by CH50 sheep erythrocyte complement lysis assay in human serum. UHRA, PEI, or protamine (final polymer concentration of 1 or 2 mg/mL) was added to human serum diluted with GVB2+ buffer (CompTech) and incubated for 1 hour at 37°C. Samples were then diluted 1:3 in GVB2+ buffer and incubated with an equal volume of antibody-sensitized sheep erythrocytes (CompTech) for 1 hour at 37°C. The reaction was stopped by addition of 300 μL of cold GVB-EDTA to each sample. Intact antibody-sensitized sheep erythrocytes were collected at 8000 rpm for 3 minutes, and supernatants were sampled. Percentage of sheep erythrocyte lysis was calculated by using average absorbance at 540 nm (A540) values.
Inhibition of thrombin binding to polyP
Streptavidin-coated 96-well plates (Corning) were incubated with 20 µM biotinylated polyP (monomer concentration, prepared as described11 ) diluted in 50 mM tris(hydroxymethyl)aminomethane-HCl (pH 7.4), 1% bovine serum albumin, 0.05% NaN3, and 0.05% polyoxyethylene (20) sorbitan monolaurate (Tween-20) for 3 hours at room temperature. Wells were then washed with 1 M LiCl and water and incubated with 40 nM bovine α-thrombin (Enzyme Research Laboratories) plus varying concentrations of UHRA inhibitors in 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) NaOH (pH 7.4), 50 mM NaCl, 1.4 mM CaCl2, 0.5 mM MgCl2, 0.1% bovine serum albumin, 0.05% Tween-20, and 0.05% NaN3 for 1 hour at room temperature. After washing with 20 mM HEPES NaOH (pH 7.4), 0.05% Tween-20, and 0.05% NaN3, the amount of thrombin bound to polyP was quantified by monitoring the rate of cleavage of 400 μM Sar-Pro-Arg-p-nitroanilide (Bachem) in 20 mM HEPES NaOH (pH 7.4) and 0.05% NaN3 by measuring A405 every 30 seconds for 20 minutes at room temperature in a SpectraMax Plate Reader (Molecular Devices).
Plasma clotting assays
Plasma clotting times were quantified at 37°C by using a STart4 coagulometer (Diagnostica Stago). All clotting assays used final concentrations in the clotting reactions of 25 μM liposomes (70:30 phosphatidylcholine:phosphatidylserine), 41.7 mM imidazole (pH 7.0), and 8.33 mM CaCl2. A 50-μL aliquot of a solution containing activator (polyP or polyguanylic acid), inhibitor, and liposomes was pipetted into a prewarmed coagulometer cuvette, after which 50 μL of prewarmed pooled normal plasma (George King Bio-Medical) was added and allowed to incubate for 3 minutes at 37°C. Clotting was then initiated by the addition of 50 μL of prewarmed 25 mM CaCl2. Activator concentrations of 10 μM long-chain polyP or 25 μg/mL polyguanylic acid (RNA) were chosen to give 60- to 80-second clotting times without inhibitors.
Animals
C57BL/6 mice were obtained from Harlan Laboratories. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign.
FeCl3-induced thrombosis in mouse carotid arteries
Mice were anesthetized by using an inhaled isoflurane-oxygen mixture, and UHRA compounds diluted in sterile normal saline were injected retroorbitally. The left carotid artery was exposed via a midline cervical incision and blunt dissection, and blood flow was monitored with a Doppler vascular flow probe (Transonic 0.5PSB) connected to a perivascular flowmeter (Transonic TS420). To induce thrombosis, two 1 × 2-mm pieces of filter paper (Whatman GB003) saturated with freshly prepared 5% anhydrous FeCl3 in 0.9% saline were applied to the deep and superficial surfaces of the artery. After 5 minutes, the filter papers were removed and the vessel was irrigated with saline. Blood flow was monitored from FeCl3 application for 30 minutes or until occlusion, defined as no detectable flow for 1 minute. Mice were then euthanized by cervical dislocation while still under anesthesia. Flow data were interpreted with LabScribe2 (iWorx Systems). Statistical analyses were performed by using GraphPad Prism 5. Unless otherwise noted, data throughout this study are reported as mean ± standard error of the mean.
Laser-induced thrombosis in mouse cremaster arterioles
Mice were anesthetized with intraperitoneal injection of 125 mg/kg ketamine, 12.5 mg/kg xylazine, and 0.25 mg/kg atropine sulfate. Approximately 10 minutes prior to imaging, fluorescent antibodies against platelets and fibrin were injected via the jugular vein along with either UHRA compounds or saline. One antibody recognized the GPIbβ subunit of the murine GPIb-V-IX complex (rat antibody X649 conjugated to DyLight649; Emfret Analytics) and the other recognized fibrin (mouse anti-fibrin antibody clone 59D8 [a generous gift from Hartmut Weiler] labeled with an Alexa Fluor 488 protein labeling kit; Invitrogen).
Anesthetized mice were placed on an intravital microscopy tray, and the testis and surrounding cremaster muscle were exteriorized and prepared for microscopy by stretching and pinning the tissue onto a custom-made intravital microscopy stage. The cremaster preparation was superfused with 37°C sterile 0.9% saline throughout the experiment. Fluorescent and brightfield images were captured for 2 minutes following injury by using a 532-nm pulsed laser system integrated with image capture and analysis software (VIVO Imaging System with Ablate! Photomanipulation Module; Intelligent Imaging Innovations). Up to 6 injuries were made per mouse with each subsequent injury upstream of previous ones.
Image analysis of fluorescent platelet and fibrin accumulation
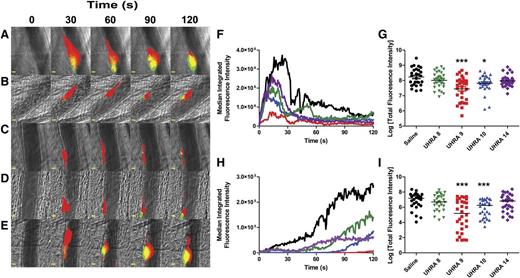
Integrated fluorescence intensity was calculated with Slidebook 5.5 (Intelligent Imaging Innovations) as reported.12 For each injury, a rectangular area was defined upstream of the injury site that included both the vessel and the surrounding tissue. The average maximal values for the Cy-5 (X649 [platelets]) and fluorescein (59D8-Alexa Fluor 488 [fibrin]) channels were used as the threshold values to create masks for both platelet and fibrin accumulation. For viewing purposes, the fluorescent pixels in Figure 1A-E and Figure 2A-E were binarized (value set to 1) if they exceeded the threshold. To determine the integrated fluorescence intensity for platelets and fibrin, only pixels above this threshold were used in data analysis (but were not binarized). In addition, a small rectangular area was defined completely within the vessel upstream of the injury to serve as a background measurement. Integrated fluorescence intensity at each time point was calculated by using the following formula: (sum intensity of the mask – [background intensity × area of the mask in pixels]). Statistical analysis was done by plotting the area under the curve (total fluorescence intensity) for each injury in a given condition and comparing the median values by using the Mann-Whitney U test. All statistical tests were performed by using GraphPad Prism 5.0.
UHRA compounds inhibit thrombus formation in mouse cremaster arterioles. (A-E) Binarized images from 1 representative injury each showing the accumulation of platelets (red) and fibrin (green) 120 seconds after laser-induced injury to the vessel wall in mice administered either (A) saline or the following UHRA compounds at 40 mg/kg: (B) UHRA-8, (C) UHRA-9, (D) UHRA-10, or (E) UHRA-14. Scale bars: 10 μm. (F-I) Statistical analyses of the effect of administering UHRA compound on thrombus formation; data are from 27 to 30 injuries to 5 mice for each condition. Median integrated fluorescence intensities (nonbinarized) were plotted vs time for accumulation of (F) platelets and (H) fibrin. In addition, the area under the curve (total fluorescence intensity) was plotted for accumulation of (G) platelets and (I) fibrin (each point represents 1 injury, plotted as log value). When median values were evaluated by the Mann-Whitney U test, both UHRA-9 and -10 significantly reduced total accumulation of platelets and fibrin compared with control. *P < .05; ***P < .0005. Brightfield and fluorescent images of arterioles were acquired with a Zeiss Axioplan microscope equipped with a Lumencore 4-LED light engine, an ×20 water immersion lens (Zeiss W-Plan APOCHROMAT ×20/1.0 NA), and a Rolera EM-C2 EMCCD Camera (Q-Imaging). Endothelial injury to the vascular wall of 50- to 70-µm diameter arterioles that resulted in thrombus formation was effected by using a 532-nm pulsed-laser system integrated with the image capture and analysis software (VIVO Imaging System with Ablate! Photomanipulation Module; Intelligent Imaging Innovations). Fluorescence images were acquired continuously, platelet fluorescence was imaged with a Cy-5 filter and 15 ms exposure, and fibrin was imaged with a fluorescein filter set and 10 ms exposure. Brightfield images were captured with a 10 ms exposure periodically (1 image every 100 captures).
UHRA compounds inhibit thrombus formation in mouse cremaster arterioles. (A-E) Binarized images from 1 representative injury each showing the accumulation of platelets (red) and fibrin (green) 120 seconds after laser-induced injury to the vessel wall in mice administered either (A) saline or the following UHRA compounds at 40 mg/kg: (B) UHRA-8, (C) UHRA-9, (D) UHRA-10, or (E) UHRA-14. Scale bars: 10 μm. (F-I) Statistical analyses of the effect of administering UHRA compound on thrombus formation; data are from 27 to 30 injuries to 5 mice for each condition. Median integrated fluorescence intensities (nonbinarized) were plotted vs time for accumulation of (F) platelets and (H) fibrin. In addition, the area under the curve (total fluorescence intensity) was plotted for accumulation of (G) platelets and (I) fibrin (each point represents 1 injury, plotted as log value). When median values were evaluated by the Mann-Whitney U test, both UHRA-9 and -10 significantly reduced total accumulation of platelets and fibrin compared with control. *P < .05; ***P < .0005. Brightfield and fluorescent images of arterioles were acquired with a Zeiss Axioplan microscope equipped with a Lumencore 4-LED light engine, an ×20 water immersion lens (Zeiss W-Plan APOCHROMAT ×20/1.0 NA), and a Rolera EM-C2 EMCCD Camera (Q-Imaging). Endothelial injury to the vascular wall of 50- to 70-µm diameter arterioles that resulted in thrombus formation was effected by using a 532-nm pulsed-laser system integrated with the image capture and analysis software (VIVO Imaging System with Ablate! Photomanipulation Module; Intelligent Imaging Innovations). Fluorescence images were acquired continuously, platelet fluorescence was imaged with a Cy-5 filter and 15 ms exposure, and fibrin was imaged with a fluorescein filter set and 10 ms exposure. Brightfield images were captured with a 10 ms exposure periodically (1 image every 100 captures).
UHRA-10 inhibits thrombus formation in mouse cremaster arterioles in a dose-dependent manner. (A-E) Binarized images from 1 representative injury each showing the effects on accumulation of platelets (red) and fibrin (green) 120 seconds after laser-induced injury to the vessel wall in mice administered either (A) saline or UHRA-10 at (B) 10 mg/kg, (C) 20 mg/kg, (D) 40 mg/kg, or (E) 80 mg/kg. Scale bars: 10 μm. (F-I) Statistical analyses of the dose-dependent attenuation of thrombus formation by UHRA-10; data are from 27 to 30 injuries to 5 mice for each condition. Median integrated fluorescence intensities (nonbinarized) were plotted vs time for accumulation of (F) platelets and (H) fibrin. In addition, the area under the curve (total fluorescence intensity) for each individual injury was plotted for accumulation of (G) platelets and (I) fibrin (each point represents 1 injury, plotted as log value). Note: data for saline control and UHRA at 40 mg/kg are in common with the data from Figure 1 and are therefore repeated here in (A) and (D), and the blue lines and data points in (F-I). Median values were compared with saline control for statistical significance by Mann-Whitney U test. UHRA-10 significantly reduced platelet accumulation at doses of 20 and 40 mg/kg and significantly reduced fibrin accumulation at doses of 40 and 80 mg/kg. *P < .05; **P < .005. Data were captured and analyzed as in Figure 1.
UHRA-10 inhibits thrombus formation in mouse cremaster arterioles in a dose-dependent manner. (A-E) Binarized images from 1 representative injury each showing the effects on accumulation of platelets (red) and fibrin (green) 120 seconds after laser-induced injury to the vessel wall in mice administered either (A) saline or UHRA-10 at (B) 10 mg/kg, (C) 20 mg/kg, (D) 40 mg/kg, or (E) 80 mg/kg. Scale bars: 10 μm. (F-I) Statistical analyses of the dose-dependent attenuation of thrombus formation by UHRA-10; data are from 27 to 30 injuries to 5 mice for each condition. Median integrated fluorescence intensities (nonbinarized) were plotted vs time for accumulation of (F) platelets and (H) fibrin. In addition, the area under the curve (total fluorescence intensity) for each individual injury was plotted for accumulation of (G) platelets and (I) fibrin (each point represents 1 injury, plotted as log value). Note: data for saline control and UHRA at 40 mg/kg are in common with the data from Figure 1 and are therefore repeated here in (A) and (D), and the blue lines and data points in (F-I). Median values were compared with saline control for statistical significance by Mann-Whitney U test. UHRA-10 significantly reduced platelet accumulation at doses of 20 and 40 mg/kg and significantly reduced fibrin accumulation at doses of 40 and 80 mg/kg. *P < .05; **P < .005. Data were captured and analyzed as in Figure 1.
Mouse tail bleeding assay
Mice were anesthetized with an inhaled isoflurane-oxygen mixture and placed on a heated surgical tray. UHRA compound, heparin, or saline alone was injected retroorbitally, and the tail tip was immersed for 5 minutes in a 15-mL plastic test tube filled with 37°C PBS. The distal 2 to 4 mm of tail was then transected with a razor blade and re-immersed in 37°C PBS for 10 minutes. Bleeding time was measured with a stopwatch for the entire 10 minutes, after which the blood samples were pelleted at 500g for 10 minutes at room temperature, and the pellet was resuspended in 5 mL Drabkin’s Reagent (Sigma) and incubated at room temperature for 15 minutes. Amount of hemoglobin lost was quantified by comparing the absorbance of the samples at 540 nm to a standard curve of bovine hemoglobin in Drabkin’s reagent.
Results
Synthesis of nontoxic polyP inhibitors
In order to have clinically useful polyP inhibitors, we needed less toxic compounds than the cationic polymers and proteins previously used in proof-of-principle studies.6,7 To address this problem, we tested a library of UHRA compounds for their ability to inhibit polyP activity in vitro and in vivo. The inclusion of a shell of short-chain PEG moieties in the UHRA compounds, together with the use of tertiary rather than primary amines, is designed to make these compounds much less toxic than previously studied polyP inhibitors containing multiple primary amines (Figure 3A).
Design and structure of UHRA compounds and efficacy in blocking thrombin binding to polyP. (A) Representative structure of a typical UHRA scaffold (in this case, for UHRA-10) containing a dendritic polyglycerol core bearing the randomly distributed polyP-binding groups (R) and an outer shell of short-chain polyethylene glycols. The molecular weight and number of R groups was varied to generate the other UHRAs. (B) Fifty percent inhibitory concentration (IC50) values for the ability of 16 UHRA compounds to inhibit thrombin binding to immobilized polyP. Arrows indicate the 4 compounds selected for further study: UHRA-8 (R = 24; 23 kDa), UHRA-9 (R = 16; 16 kDa), UHRA-10 (R = 11; 10 kDa), and UHRA-14 (R = 7; 10 kDa).
Design and structure of UHRA compounds and efficacy in blocking thrombin binding to polyP. (A) Representative structure of a typical UHRA scaffold (in this case, for UHRA-10) containing a dendritic polyglycerol core bearing the randomly distributed polyP-binding groups (R) and an outer shell of short-chain polyethylene glycols. The molecular weight and number of R groups was varied to generate the other UHRAs. (B) Fifty percent inhibitory concentration (IC50) values for the ability of 16 UHRA compounds to inhibit thrombin binding to immobilized polyP. Arrows indicate the 4 compounds selected for further study: UHRA-8 (R = 24; 23 kDa), UHRA-9 (R = 16; 16 kDa), UHRA-10 (R = 11; 10 kDa), and UHRA-14 (R = 7; 10 kDa).
UHRA compounds inhibit polyP-thrombin binding and polyP-initiated plasma clotting in vitro
We first screened a panel of UHRA compounds for ability to inhibit thrombin binding to immobilized polyP, a high-throughput method for identifying polyP blockers.6 UHRA compounds 1 to 16 were individually screened in this assay (Figure 3B; full data are available in supplemental Table 1). Eleven of the UHRA compounds had 50% inhibitory concentration (IC50) values of ≤10 nM for inhibiting thrombin binding to polyP, of which we selected 4 for further testing: UHRA-8, -9, -10, and -14, which all inhibited thrombin binding to polyP with IC50 values in the 5- to 8-nM range (Table 1).
In vitro inhibition of polyP activity by selected UHRA compounds
| Compound . | Size (kDa) . | No. of R groups . | Thrombin binding . | Plasma clotting assay . | ||||
|---|---|---|---|---|---|---|---|---|
| IC50 . | ECdouble polyP . | ECdouble RNA . | ||||||
| nM . | ng/mL . | nM . | µg/mL . | µM . | µg/mL . | |||
| UHRA-8 | 23 | 24 | 5.4 ± 1.8 | 124 ± 41 | 52 ± 15 | 1.20 ± 0.34 | 0.62 ± 0.21 | 14 ± 4.8 |
| UHRA-9 | 16 | 16 | 7.6 ± 2.0 | 122 ± 32 | 80 ± 16 | 1.28 ± 0.26 | 1.9 ± 0.59 | 30 ± 9.4 |
| UHRA-10 | 10 | 11 | 7.3 ± 3.7 | 73 ± 37 | 132 ± 38 | 1.32 ± 0.38 | 2.0 ± 0.58 | 20 ± 5.8 |
| UHRA-14 | 10 | 7 | 6.6 ± 2.4 | 66 ± 24 | 92 ± 13 | 0.92 ± 0.13 | 2.2 ± 0.65 | 22 ± 6.5 |
| Compound . | Size (kDa) . | No. of R groups . | Thrombin binding . | Plasma clotting assay . | ||||
|---|---|---|---|---|---|---|---|---|
| IC50 . | ECdouble polyP . | ECdouble RNA . | ||||||
| nM . | ng/mL . | nM . | µg/mL . | µM . | µg/mL . | |||
| UHRA-8 | 23 | 24 | 5.4 ± 1.8 | 124 ± 41 | 52 ± 15 | 1.20 ± 0.34 | 0.62 ± 0.21 | 14 ± 4.8 |
| UHRA-9 | 16 | 16 | 7.6 ± 2.0 | 122 ± 32 | 80 ± 16 | 1.28 ± 0.26 | 1.9 ± 0.59 | 30 ± 9.4 |
| UHRA-10 | 10 | 11 | 7.3 ± 3.7 | 73 ± 37 | 132 ± 38 | 1.32 ± 0.38 | 2.0 ± 0.58 | 20 ± 5.8 |
| UHRA-14 | 10 | 7 | 6.6 ± 2.4 | 66 ± 24 | 92 ± 13 | 0.92 ± 0.13 | 2.2 ± 0.65 | 22 ± 6.5 |
Results are 50% inhibitory concentration (IC50) for inhibiting thrombin binding to immobilized polyP (n = 5); and ECdouble (concentration needed to double the clotting time) in plasma clotting assays initiated with either 10 μM long-chain polyP (ECdouble polyP, n = 3) or 25 µg/mL polyguanylic acid (ECdouble RNA, n = 3). Data are reported in terms of both molarity and mass/volume ± standard error of the mean.
Because the thrombin binding assay is performed in the absence of plasma proteins that might compete for binding to polyP, we also examined the ability of the 4 selected compounds to inhibit contact activation in a modified activated partial thromboplastin time clotting assay initiated by long-chain polyP (>1000 phosphates per chain) or polyguanylic acid (RNA). All 4 compounds doubled the polyP-initiated clotting times in the 50- to 150-nM range and the RNA-initiated clotting times in the 1- to 2-μM range (Table 1). In contrast, even very high concentrations of UHRAs showed only minimal prolongation of the plasma clotting time initiated by tissue factor (supplemental Figure 1).
Both the thrombin-polyP binding assays and clotting assays were performed at ionic strengths lower than that of plasma. Therefore, we used isothermal titration calorimetry to determine the parameters for binding polyP to UHRA-8, -9, -10, and -14 at physiologic ionic strength (supplemental Figure 2) to better predict the concentrations of these UHRA compounds that might be needed to inhibit polyP function in blood. UHRA-8, -9, -10, and -14 bound to polyP with Kd values in the 0.7- to 2.2-µM range (supplemental Table 2).
Biocompatibility of UHRA compounds compared with previously identified polyP inhibitors
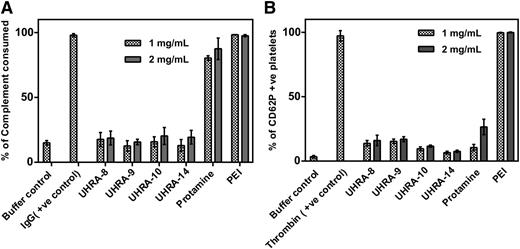
To examine whether the unique structure of the UHRA compounds allowed them to inhibit polyP with reduced toxic side effects, we investigated the interaction of UHRAs with blood components in comparison with previously reported polyP inhibitors such as PEI and protamine sulfate.6 Compared with buffer controls, complement activation was undetectable with UHRAs even at 2 mg/mL (Figure 4A). Conversely, PEI and protamine strongly activated complement. We also studied platelet activation by measuring CD62P expression in PRP. UHRAs exhibited lower levels of platelet activation (10% to 15%) compared with protamine and PEI, which activated platelets by about 30% and 100%, respectively, when tested at the highest dose (Figure 4B).
Biocompatibility of UHRAs compared with other polyP inhibitors. (A) The ability of UHRA compounds, protamine, and PEI (tested at 1 or 2 mg/mL) to activate complement in human serum was measured by sheep erythrocyte complement consumption assay. Heat-aggregated human immunoglobulin G (IgG; 1 mg/mL) and PBS were the positive and negative controls, respectively. UHRAs did not activate complement compared with buffer controls, whereas protamine and PEI showed high levels of complement activation. (B) The ability of UHRA compounds, protamine, and PEI (tested at 1 and 2 mg/mL) to activate platelets in human PRP was measured by flow cytometry for expression of platelet activation marker CD62P. Bovine thrombin (at 1 IU/mL) was used as the positive control, and PBS alone was used as the negative control (buffer control). UHRAs showed low levels of platelet activation compared with PEI. +ve, positive.
Biocompatibility of UHRAs compared with other polyP inhibitors. (A) The ability of UHRA compounds, protamine, and PEI (tested at 1 or 2 mg/mL) to activate complement in human serum was measured by sheep erythrocyte complement consumption assay. Heat-aggregated human immunoglobulin G (IgG; 1 mg/mL) and PBS were the positive and negative controls, respectively. UHRAs did not activate complement compared with buffer controls, whereas protamine and PEI showed high levels of complement activation. (B) The ability of UHRA compounds, protamine, and PEI (tested at 1 and 2 mg/mL) to activate platelets in human PRP was measured by flow cytometry for expression of platelet activation marker CD62P. Bovine thrombin (at 1 IU/mL) was used as the positive control, and PBS alone was used as the negative control (buffer control). UHRAs showed low levels of platelet activation compared with PEI. +ve, positive.
Cationic PAMAM dendrimers have been identified as proof-of-principle polyP blockers,6,7 although they are reported to have significant toxicity, including the ability to cause fibrin(ogen) aggregation and induce a state similar to disseminated intravascular coagulation.8,9,13,14 Furthermore, the in vivo toxicity of amine-terminated PAMAM dendrimers increases with generation (reviewed by Sadekar et al14 ), which is unfortunate because their effectiveness in blocking polyP also increases with generation.6,7 We examined the ability of UHRA-8, -9, -10, and -14 versus cationic PAMAM dendrimers (generations 1 to 7) to induce fibrinogen aggregation (supplemental Figure 3). Even when tested at 1.5 mg/mL, none of the UHRA compounds showed evidence of inducing fibrinogen aggregation. Conversely, generations 3 to 7 PAMAM dendrimers caused fibrinogen aggregation at doses of 0.2 to 1 mg/mL.
In vivo toxicity studies demonstrated that UHRA-10 was well tolerated in mice after intravenous injection, with no adverse effects up to 200 mg/kg (the maximum injected dose) and essentially identical body weights over a 14-day period relative to saline-injected control mice (supplemental Figure 4A). In addition, mice injected with 100 or 200 mg/kg UHRA-10 had serum lactate dehydrogenase levels within normal limits (supplemental Figure 4B). No abnormalities were observed in necropsy performed at day 14, with histopathology analyses showing no evidence of tissue damage, necrosis, or inflammation (supplemental Figure 4C), all consistent with the nontoxic nature of the UHRAs.
UHRA compounds are antithrombotic in 2 mouse models of arterial thrombosis
To test the antithrombotic effectiveness of UHRA compounds in vivo, we used 2 mouse models of thrombus formation: laser-induced injury to cremaster arterioles and FeCl3-induced injury to carotid arteries. Initially, 40 mg/kg of UHRA-8, -9, -10, or -14 was administered intravenously to mice after which cremaster arterioles were injured (Figure 1). UHRA-9 and -10 significantly reduced the accumulation of both platelets and fibrin, with UHRA-9 resulting in a 73% decrease in median total platelet fluorescence intensity (Figure 1C, F-G; P = .0006) and a 99% decrease in median fibrin total fluorescence intensity (Figure 1C, H-I; P = .0001) compared with saline control. UHRA-10 was similarly effective, resulting in a 63% reduction in total platelet fluorescence intensity (Figure 1D, F-G; P = .018) and an 88% reduction in fluorescent fibrin accumulation (Figure 1D, F-G; P = .0009). Although UHRA-8 (Figure 1B) and UHRA-14 (Figure 1E) reduced median platelet total fluorescence intensity by 41% and 60% (Figure 1F-G) and median fibrin total fluorescence intensity by 66% and 56% (Figure 1 H-I), respectively, these decreases were not statistically significant from saline controls. We then varied the dose of UHRA-10 to establish its range of effectiveness (Figure 2). UHRA-10 significantly reduced median total platelet fluorescence at doses of 20 and 40 mg/kg (Figure 2C-D, F-G; P = .001 and P = .018, respectively) with a maximum inhibition of 73% at the 20-mg/kg dose. Median total fibrin fluorescence was significantly inhibited at doses of 40 and 80 mg/kg (Figure 2D-E, H-I; P = .0009 and P = .0013, respectively), with a maximum inhibition of 94% at the 80-mg/kg dose.
We also examined the ability of UHRA-10 to inhibit mouse carotid artery thrombosis induced by topical application of FeCl3 (Figure 5) and found that a dose of 100 mg/kg UHRA-10 performed as well as a dose of 200 U/kg unfractionated heparin, because both treatments significantly increased the median patency time (P = .0004 for UHRA-10 and P = .007 for heparin). A dose of 200 mg/kg UHRA-10 was as effective as 1000 U/kg unfractionated heparin in completely blocking thrombus formation for 30 minutes.
UHRA-10 delays time to occlusion in a mouse carotid artery model of thrombosis. Artery patency was monitored by Doppler flow probe following induction of FeCl3-mediated injury and plotted here vs time in black for saline control, orange for unfractionated heparin, and blue for UHRA-10. Both heparin and UHRA-10 significantly delayed median time to occlusion in a dose-dependent manner (P < .0001). Heparin at 200 U/kg was not significantly more effective than UHRA-10 at 100 mg/kg at maintaining artery patency (P = .85), whereas both treatment conditions significantly increased median patency time vs saline control (P = .0004 for UHRA-10 and P = .007 for heparin). UHRA-10 at 200 mg/kg or heparin at 1000 U/kg resulted in 100% patency over the 30-minute period for all mice (n = 7 for all conditions). Statistical significance was assessed by log-rank analysis.
UHRA-10 delays time to occlusion in a mouse carotid artery model of thrombosis. Artery patency was monitored by Doppler flow probe following induction of FeCl3-mediated injury and plotted here vs time in black for saline control, orange for unfractionated heparin, and blue for UHRA-10. Both heparin and UHRA-10 significantly delayed median time to occlusion in a dose-dependent manner (P < .0001). Heparin at 200 U/kg was not significantly more effective than UHRA-10 at 100 mg/kg at maintaining artery patency (P = .85), whereas both treatment conditions significantly increased median patency time vs saline control (P = .0004 for UHRA-10 and P = .007 for heparin). UHRA-10 at 200 mg/kg or heparin at 1000 U/kg resulted in 100% patency over the 30-minute period for all mice (n = 7 for all conditions). Statistical significance was assessed by log-rank analysis.
Antithrombotic doses of UHRA-10 caused less bleeding compared with unfractionated heparin
To test whether UHRA-10 caused less bleeding than heparin, we used a mouse tail bleeding model to compare treatment with 50-, 100-, and 200-mg/kg doses of UHRA-10 to treatment either with saline alone or with 200 or 1000 U/kg doses of unfractionated heparin (Figure 6). Mice treated with a 200-U/kg dose of heparin had significantly longer bleeding times compared with saline controls or with mice treated with 50- or 100-mg/kg doses of UHRA-10 (P < .0001; Figure 6A). As expected, heparin-treated mice also lost significantly more hemoglobin due to bleeding than did saline-treated mice (P = .022; Figure 6B). Although mice treated with 50 or 100 mg/kg UHRA-10 lost less hemoglobin (10.8 ± 2.4 and 7.9 ± 1.4 mg, respectively) than heparin-treated mice (15.7 ± 4.4 mg), the differences were not statistically significant (P = .16 and P = .11, respectively). Mice treated with the highest dose of UHRA-10 (200 mg/kg) had significantly shorter bleeding times (P = .047) but no significant difference in hemoglobin lost (P = .55) compared with mice treated with 1000 U/kg heparin (Figure 6).
Antithrombotic doses of UHRA-10 caused less bleeding than did unfractionated heparin in a mouse tail bleeding model. (A) Bleeding times. Mice treated with 200 U/kg unfractionated heparin had significantly longer tail bleeding times than did either saline control mice or mice treated with 50 or 100 mg/kg UHRA-10. Similarly, mice treated with 1000 U/kg heparin had significantly longer bleeding times than did mice treated with 200 mg/kg UHRA-10. (B) Blood loss (quantified as milligrams of hemoglobin collected during 30 minutes). Mice treated with either 200 or 1000 U/kg heparin had significantly higher hemoglobin loss than did mice treated with saline. Mice treated with 1000 U/kg heparin had significantly more hemoglobin loss than did mice treated with 50 or 100 mg/kg UHRA-10. Mice treated with 1000 U/kg heparin had no significant difference in hemoglobin loss compared with mice treated with 200 mg/kg UHRA-10. Statistical significance was assessed by individual Student t tests. *P < .05; **P < .005; ***P < .0005.
Antithrombotic doses of UHRA-10 caused less bleeding than did unfractionated heparin in a mouse tail bleeding model. (A) Bleeding times. Mice treated with 200 U/kg unfractionated heparin had significantly longer tail bleeding times than did either saline control mice or mice treated with 50 or 100 mg/kg UHRA-10. Similarly, mice treated with 1000 U/kg heparin had significantly longer bleeding times than did mice treated with 200 mg/kg UHRA-10. (B) Blood loss (quantified as milligrams of hemoglobin collected during 30 minutes). Mice treated with either 200 or 1000 U/kg heparin had significantly higher hemoglobin loss than did mice treated with saline. Mice treated with 1000 U/kg heparin had significantly more hemoglobin loss than did mice treated with 50 or 100 mg/kg UHRA-10. Mice treated with 1000 U/kg heparin had no significant difference in hemoglobin loss compared with mice treated with 200 mg/kg UHRA-10. Statistical significance was assessed by individual Student t tests. *P < .05; **P < .005; ***P < .0005.
Discussion
PolyP inhibitors have recently emerged as candidates for antithrombotics with a novel mode of action that differs dramatically from that of conventional antithrombotics.6,7 We now report the successful application of a new molecular scaffold for developing antithrombotic agents that target polyP. UHRAs are dendrimer-like compounds engineered to contain multiple positively charged, branched tertiary amines shielded by a protective layer of short-chain PEG groups. When we examined a library of 16 UHRA compounds containing from 1 to 33 R groups (with each R group containing 4 tertiary amines), we found that their ability to inhibit thrombin-polyP binding was influenced by the number of R groups in the compound, with the most potent inhibitors requiring the presence of ≥5 such R groups. We chose 4 highly potent UHRA compounds (UHRA-8, -9, -10, and -14, ranging from 7 to 24 R groups per molecule) for more detailed studies. These 4 compounds strongly inhibited clotting of plasma initiated by both long-chain polyP and RNA (another procoagulant polyanion15 ), with a 12- to 24-fold higher potency toward polyP than RNA.
When tested in a cremaster arteriole thrombosis model, treatment with all 4 UHRA compounds resulted in lower median levels of accumulation of platelets and fibrin compared with saline-treated controls, although the reductions were statistically significant only for UHRA-9 and -10. UHRA-14 has the same molecular weight as UHRA-10 but has a lower charge density (with 7 R groups in the former and 11 in the latter), and indeed, UHRA-14 was a less effective antithrombotic in vivo than was UHRA-10. Interestingly, UHRA-8, which was consistently the best polyP inhibitor in vitro, did not perform as well as UHRA-9 or -10 in vivo.
Higher concentrations of UHRA-10 were needed to inhibit thrombus formation in the carotid artery thrombosis model, a finding consistent with previous studies reporting that complete inhibition of arterial thrombosis caused by injury with 5% FeCl3 necessitates antithrombotic therapy at doses high enough to induce bleeding problems.16 Conversely, laser-induced thrombosis in mouse cremaster arterioles has been shown to be sensitive to intervention at more clinically relevant levels of antithrombotic therapy.12,17
In previous toxicity studies10 as well as those reported here (supplemental Figure 4), UHRAs did not show hemolysis and red blood cell aggregation even at 5 mg/mL, whereas protamine and PEI induced significant hemolysis. UHRA compounds also did not show any effect on thrombin generation in human PRP; furthermore, UHRAs were well tolerated in mice after intravenous injection with no adverse effects up to 200 mg/kg (the maximum injected dose) in vivo, and the maximum tolerated dose was not reached.10 Conversely, protamine was tolerated up to only 20 mg/kg. Biodistribution studies using tritiated UHRA (23 kDa) gave a plasma half-life of about 40 minutes with very low accumulation in vital organs (about 8% of the initial dose in the liver and 2% in spleen, kidney, heart, and lung after 48 hours) and was cleared mainly via the kidneys.10 All these data demonstrated the safety of the UHRAs in vivo when compared with the conventional cationic polymers.
This low toxicity profile for UHRA compounds makes them more attractive for clinical use than polyP inhibitors such as polyethylenimine, protamine, polymyxin B, or PAMAM dendrimers.6,7 Polymyxin B has well-documented toxicity in humans.18 Protamine has demonstrated toxicity as well, including anticoagulant effects in the absence of heparin19,20 and the ability to precipitate fibrinogen at high concentrations.21 Cationic PAMAM dendrimers also interact with and precipitate fibrinogen in solution,9 which was confirmed in this study. UHRA compounds showed no signs of these adverse interactions at concentrations of up to 1.5 mg/mL.
The nontoxic and modular nature of UHRA compounds like the ones investigated in this study makes them attractive candidates for the development of clinical antithrombotic agents with a novel mode of action. Although we cannot conclusively say that the antithrombotic nature of these compounds is entirely based on their ability to bind to polyP and inhibit its role in thrombus formation in vivo, doses of the compound that are well-tolerated in mice were as effective as heparin in inhibiting thrombosis with fewer bleeding side effects. Although heparin and heparin derivatives are some of the most widely used antithrombotics today, they have well-documented drawbacks that extend even beyond the bleeding risks.22,23
Future generations of the compounds might be developed that have more or less specificity for polyP versus other anionic polymers such as extracellular nucleic acids, which have also been implicated in pathologic thrombosis.7,24 This could lead to a variety of antithrombotic therapeutics that could treat the different causes of thrombosis in individual patients more efficiently. An intriguing possibility along these lines could be the use of polyP inhibitors in sepsis and disseminated intravascular coagulation, in which not only platelet polyP but also long-chain polyP from infectious microorganisms might play a role in pathologic thrombus formation. Microbial (long-chain) polyP is orders of magnitude more effective than platelet polyP at triggering the contact pathway of blood clotting and can induce multiple inflammatory reactions.25-29
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Benjamin F. L. Lai at the Centre for Blood Research for technical support.
This work was supported by Predoctoral Fellowship 13PRE14550007 from the American Heart Association (R.J.T.), contract R01 HL047014 from the National Heart, Lung and Blood Institute of the National Institutes of Health (J.H.M.), and the Canadian Institutes of Health Research and Natural Sciences and Engineering Council of Canada (J.N.K.). J.N.K. received the Michael Smith Foundation for Health Research Career Scholar Award.
Authorship
Contribution: R.J.T., R.A.S., and M.T.K. designed and carried out experiments, analyzed results, and edited the manuscript; and J.N.K. and J.H.M. designed experiments, analyzed results, and edited the manuscript.
Conflict-of-interest disclosure: The authors of this study are coinventors on pending patent applications for use of UHRA compounds as antithrombotic drugs and for clinical and diagnostic uses of polyphosphate.
Correspondence: James H. Morrissey, Department of Biochemistry, University of Illinois at Urbana-Champaign, 323 Roger Adams Laboratory, MC-712, 600 S Mathews Ave, Urbana, IL 61801; e-mail: jhmorris@illinois.edu.