In this issue of Blood, Romain et al demonstrate that natural killer (NK) cell–mediated killing of tumor cells coated with the Fc-optimized CD33 antibody DLE-HuM195 reveals a distinct kinetic profile.1 The presented work gives important novel insights into the mechanism of effector cell–mediated target cell killing triggered by Fc-engineered antibodies and explains how they achieve a higher antibody-dependent cell-mediated cytotoxicity (ADCC) potency than native immunoglobulin G1 (IgG1) antibodies. Using time-lapse imaging microscopy in nanowell grids (TIMING), the authors were able to demonstrate at the single-cell level that antibody Fc engineering improves frequency and promotes kinetic boosting of serial killing mediated by NK cells.
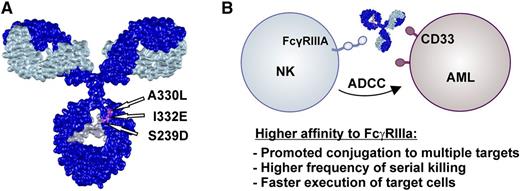
(A) Romain and colleagues generated the CD33-specific Fc-engineered antibody DLE-HuM195 by introducing 3 amino acid exchanges (S239D-A330L-I332E) in the CH2 domain of HuM195. This Fc variant has been described by Lazar et al and results in enhanced Fc affinity to FcγRIIIa and reduced C1q binding activity. (B) The improved FcγRIIIa binding affinity of DLE-HuM195 resulted in stronger ADCC activity compared with the nonengineered counterpart. Using TIMING, the authors were able to demonstrate at the single-cell level that antibody Fc engineering improves frequency and promotes kinetic boosting of serial killing mediated by NK cells. The IgG model structure is based on a protein data bank file kindly provided by Mike Clark (http://www.path.cam.ac.uk/∼mrc7/).
(A) Romain and colleagues generated the CD33-specific Fc-engineered antibody DLE-HuM195 by introducing 3 amino acid exchanges (S239D-A330L-I332E) in the CH2 domain of HuM195. This Fc variant has been described by Lazar et al and results in enhanced Fc affinity to FcγRIIIa and reduced C1q binding activity. (B) The improved FcγRIIIa binding affinity of DLE-HuM195 resulted in stronger ADCC activity compared with the nonengineered counterpart. Using TIMING, the authors were able to demonstrate at the single-cell level that antibody Fc engineering improves frequency and promotes kinetic boosting of serial killing mediated by NK cells. The IgG model structure is based on a protein data bank file kindly provided by Mike Clark (http://www.path.cam.ac.uk/∼mrc7/).
Today, antibody therapy is an established treatment option in cancer therapy. Monoclonal antibodies such as rituximab, which targets the CD20 antigen on various B-cell malignancies, have significantly improved the clinical outcome of tumor patients. Unfortunately, not all patients can benefit from a generally well-tolerated antibody therapy. For example, antibody-based therapeutic approaches in acute myeloid leukemia (AML) using unconjugated monoclonal antibodies have not been clinically approved at the moment. CD33, a candidate antigen in targeting AML, is significantly expressed in about 85% to 90% of cases and is considered a promising target structure for developing effective antibody-based AML therapies. Lintuzumab (HuM195), a humanized CD33 antibody, had observable efficacy in patients with advanced AML, but the antileukemic activity was confined to patients with low disease burden. Recent data from a phase 1 study suggested that prolonged exposure to lintuzumab results in greater therapeutic activity. The addition of lintuzumab to salvage induction chemotherapy was safe, but it did not result in a significant improved survival in patients with refractory/relapsed AML.2 Therefore, antibody engineering to enhance selected antibody effector functions of HuM195 as suggested by Romain and colleagues may improve CD33-directed antibody therapy.
Antibodies are able to mediate antitumor activity by various effector mechanisms, eg, directly, by inhibiting proliferation or triggering tumor cell apoptosis, or indirectly, by recruiting the complement system or immune effector cells to induce ADCC or phagocytosis. Results from elegant animal models suggested an important role for Fc receptor (FcR)-expressing immune effector cells in antibody therapy. Knockout of expression or signaling mediated by activating FcR resulted in loss of therapeutic activity by monoclonal antibodies.3,4 The importance of FcR engagement is further supported by analyses of certain FcγR polymorphisms in patients treated with therapeutic antibodies. In different studies, patients with homozygous expression of the FcγRIIIa-V158 allele and/or the FcγRIIa-H131 allele demonstrated higher response rates and prolonged overall survival.5 FcγRIIIa-V158 binds human IgG1-Fc with higher affinity than FcγRIIIa-F158, resulting in enhanced ADCC activity. Based on these data, Fc engineering to enhance recruitment of FcγRIIIa-positive effector cells such as NK cells or macrophages was suggested as a promising strategy to improve antibody therapy.6
It has been repeatedly shown that higher-affinity FcγRIIIa binding results in stronger ADCC activity with NK cells, but the precise cellular events leading to the observed effect remained elusive. Romain and colleagues generated an Fc-engineered CD33 antibody variant DLE-HuM195 by introducing 3 amino acid exchanges (S239D-A330L-I332E = DLE) in the CH2 domain (see figure) and used TIMING to analyze NK cell-mediated killing of antibody-coated target cells at the single-cell level.1 This technique allowed precise kinetic profiling of target cell lysis triggered by Fc-engineered vs native IgG1 antibodies. These experiments revealed that target cells coated with the optimized antibody variant are more rapidly lysed by NK cells by promoting simultaneous conjugates to multiple targets and inducing apoptosis in twice the number of target cells within the same time period compared with wild-type antibodies. Because Fc-engineering approaches by amino acid exchange as applied by the authors often do not selectively enhance affinity to FcγRIIIa but result in altered binding affinities to other Fcγ receptors (eg, FcγRI, FcγRIIa, FcγRIIIb) expressed on macrophages or granulocytes, analysis of the kinetics of target cell killing by other candidate effector populations will be interesting. In other model systems enhanced FcγRIII binding affinity by Fc glycoengineering has been correlated with reduced granulocyte-mediated ADCC and enhanced antibody-dependent cell-mediated phagocytosis.7,8
From a clinical perspective, antibodies engineered for higher-affinity FcγRIIIa binding, like obinutuzumab, showed promising activity in clinic trials, but corresponding clinical data with a nonengineered counterpart are not available, making conclusions on the contribution of Fc engineering to the therapeutic effects difficult.9 In a preclinical nonhuman primate model, the cytolytic potential of an Fc-engineering strategy similar to that applied by Romain and colleagues in the background of an antibody directed against CD19 was convincingly demonstrated. In this model, the Fc-optimized antibody (MOR208, Xmab5574) efficiently depleted B cells, whereas its nonengineered counterpart almost lacked any B-cell–depleting activity.10
The generation and initial mechanistic characterization of the Fc-optimized DLE-HuM195 antibody variant sheds light on the kinetics of NK cell–mediated killing of tumor cells triggered by Fc-engineered antibodies and represents a first step toward a more effective antibody-based therapy in AML.
Conflict-of-interest disclosure: The authors declare no competing financial interests.