In this issue of Blood, Wang et al elegantly show that telomere shortening results in DNA damage that induces apoptosis and senescence in quiescent hematopoietic stem cells (HSCs).1
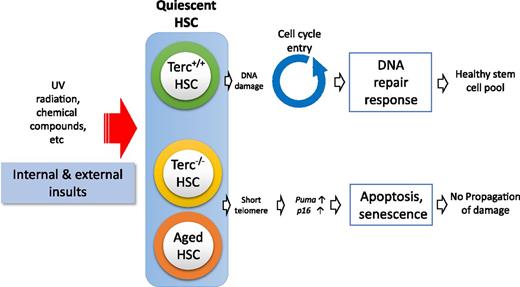
Schematic representation showing quiescent HSC response to cellular insults. Terc−/− HSCs and aged HSCs are inhibited from entering the cell cycle because of induction of senescence and apoptosis related to short telomeres.
Schematic representation showing quiescent HSC response to cellular insults. Terc−/− HSCs and aged HSCs are inhibited from entering the cell cycle because of induction of senescence and apoptosis related to short telomeres.
Altering the cell cycle and retaining a quiescent state protect cells from cell-intrinsic functional exhaustion and naturally produce extrinsic cellular insults.2 HSCs are thus maintained in cell cycle quiescence, enabling lifelong hematopoietic cell production.3,4 However, quiescence does not ensure that HSCs will be immortal. Organismal aging is reflected on the cellular level as stem cell potentials of HSCs decline with age. The mechanisms of how aged HSCs are functionally defective have recently started to be uncovered.
HSCs exhibit accumulation of DNA damage induced by intrinsic and extrinsic hazards.5 Initially, it was postulated that acquisition of quiescence protected HSCs from genomic instability. Yet, recent studies indicate decreased expression of DNA damage repair pathway–associated genes in quiescent HSCs compared with cycling HSCs.6 Attenuation of the DNA damage repair pathway leads to the accumulation of DNA damage in aged quiescent HSCs, and such damage is repaired upon cycling. Aged quiescent HSCs display a slower rate to enter the cell cycle to proliferate and display a persistent replication stress postcycling.7 Mechanisms underlying HSC aging are thus linked to cell cycle status and cell cycle machinery. However, a quiescent state does not necessarily ensure protection from cellular insults and genetic instability.
Telomeres cap the ends of linear chromosomes, protecting chromosomes from degradation or fusion.8 Telomeres shorten with cell division and aging but are repaired by telomerase ribonucleoprotein complex and the shelterin complex.8 Telomerase is expressed in HSCs, and telomerase activity is essential for the maintenance of HSC potentials.9 Yet details of the mechanism underlying the effect are largely unknown. Wang et al profiled the gene expressions of HSCs and progenitor cells from third-generation Terc-deficient mice (G3mTerc−/−). They revealed that gene expression changes were confined to HSCs. More excitingly, the gene expression changes were further prominent in quiescent G3mTerc−/− HSCs rather than actively cycling HSCs. When G3mTerc−/− HSCs were stimulated to cycle, HSCs that remained quiescent significantly expressed elevated levels of senescence-associated (p16) and apoptosis-associated (Puma) genes. These data indicate that telomere shortening in quiescent HSCs activates a genetic program prior to cell cycle checkpoints, which eliminates damaged HSCs through senescence or apoptosis (see figure).
The current study indicates that genomic machinery regarding telomeres actively participates in determining the cell fate of HSCs even before they confront cell cycle checkpoints upon proliferation. Although it has been shown that DNA damage repair responses are attenuated in quiescent HSCs,6 it is still too early to conclude that quiescence is a detrimental state for the preservation of HSC functions. In fact, with regard to telomere-mediated responses, quiescent HSCs integrate a surveillance program so that damage is not propagated to HSC progeny. The current paper provokes the question of how telomere shortening and telomerase modulate effects on cell cycle entry. This will provide greater insight into the biology of HSC aging.
Conflict-of-interest disclosure: The authors declare no competing financial interests.