Key Points
IL-27 inhibits CTL cytotoxicity toward autologous platelets via decreasing granzyme B expression in ITP.
Abstract
Cytotoxic T-lymphocyte (CTL)-mediated platelet destruction and aberrant cytokine profiles play important roles in the pathogenesis of primary immune thrombocytopenia (ITP). Interleukin-27 (IL-27) has pleiotropic immunomodulatory effects. However, the effect of IL-27 on CTL activity in ITP has not been reported. In the present study, platelets from ITP patients were cultured with autologous CTLs in the presence of IL-27. We found that IL-27 could inhibit CTL-mediated platelet destruction. In these IL-27–treated CTLs, granzyme B and T-bet expression decreased significantly, whereas granzyme A, perforin, and eomesodermin were not affected. To further investigate the role of granzyme B in CTL-mediated platelet destruction, granzyme B inhibitor was added and platelet apoptosis was significantly inhibited. These results suggest that IL-27 negatively regulates CTL cytotoxicity toward platelets in ITP by decreasing granzyme B expression, which is associated with reduced T-bet expression. IL-27 may have a therapeutic role in treating ITP patients.
Introduction
Primary immune thrombocytopenia (ITP) is an autoimmune disorder characterized by low platelet counts and an increased risk of bleeding.1 Cytotoxic T-lymphocyte (CTL)-mediated platelet destruction2-5 and disturbed cytokine profiles6,7 play important roles in the pathogenesis of ITP. Interleukin-27 (IL-27), a cytokine with both pro-inflammatory and anti-inflammatory effects, plays pleiotropic roles in immunomodulation.8 Recent studies have demonstrated that IL-27 could suppress inflammatory responses in T-cell differentiation and in autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus.9-11 Our previous studies reported that the expression of IL-27 was decreased and CTL-mediated platelet destruction was increased in patients with ITP.12,13 The effect of IL-27 on CTL cytotoxicity toward autologous platelets in ITP has not been reported, although IL-27 was shown to augment the number and function of CTLs in patients with tumors,14 unlike in patients with autoimmune diseases. In the present study, we cultured platelets from ITP patients with autologous CTLs in the presence of IL-27 and found that IL-27 could inhibit CTL cytotoxicity toward autologous platelets by decreasing granzyme B expression, which was associated with reduced T-bet expression, potentially providing a novel therapeutic target for the management of ITP.
Methods
Thirty-eight ITP patients with active disease and 12 healthy volunteers were enrolled in this study between April 2013 and July 2014 in the Department of Hematology, Qilu Hospital, Shandong University.
Platelets were cultured with autologous CTLs for 4 hours. Then the supernatants and cells were harvested. Platelet apoptosis, the expression of granzyme A, granzyme B, perforin, T-bet, and eomesodermin (Eomes) were analyzed.
The experimental protocol and patients’ information are described in detail in the supplemental data on the Blood Web site.
Results and discussion
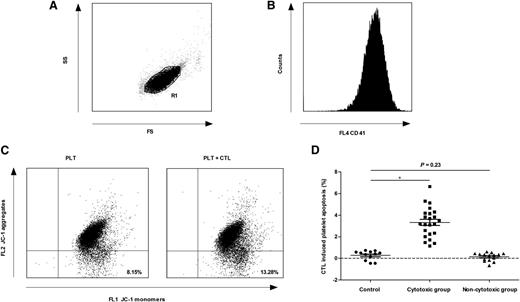
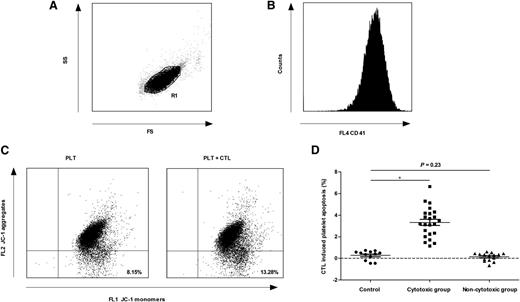
Despite the lack of solid in vivo data before now,15 several in vitro studies have provided evidence that T cell–mediated platelet destruction might be an important mechanism of thrombocytopenia in some patients with ITP.2-5,13,16 In this study, the normal range of CTL-induced platelet apoptosis was established from the mean ± 2 standard deviations of results in healthy controls. The patients with CTL-induced platelet apoptosis higher than the upper limit of normal range were assigned to the cytotoxic group (23 patients); otherwise, they were assigned to the noncytotoxic group (15 patients) (Figure 1). There was no difference between these 2 groups in the percentage of patients with a detectable platelet-specific autoantibody (supplemental Table 1 on the Blood Web site), suggesting that both CTLs and platelet-specific autoantibodies might be involved in platelet destruction in the same patient. These results further confirm the pathogenic diversity of ITP.17
CTL-induced platelet apoptosis was found in some ITP patients. Platelets were gated by (A) forward scatter (FS) and side scatter (SS), and labeled with (B) phycoerythrin-cyano dye 5–conjugated mouse anti-human CD41a. (C) The apoptosis of platelets from 1 ITP patient was 8.15% (cultured alone) and 13.28% (cultured with autologous CTLs), respectively. (D) Thirty-eight ITP patients were divided into 2 groups. CTL-induced platelet apoptosis in the cytotoxic group (23 patients) was significantly higher than that of healthy controls (3.32% ± 1.38% vs 0.27% ± 0.43%, P < .01), whereas that in the noncytotoxic group (15 patients) was not different from healthy controls (0.14% ± 0.38% vs 0.27% ± 0.43%, P = .23). *P < .01.
CTL-induced platelet apoptosis was found in some ITP patients. Platelets were gated by (A) forward scatter (FS) and side scatter (SS), and labeled with (B) phycoerythrin-cyano dye 5–conjugated mouse anti-human CD41a. (C) The apoptosis of platelets from 1 ITP patient was 8.15% (cultured alone) and 13.28% (cultured with autologous CTLs), respectively. (D) Thirty-eight ITP patients were divided into 2 groups. CTL-induced platelet apoptosis in the cytotoxic group (23 patients) was significantly higher than that of healthy controls (3.32% ± 1.38% vs 0.27% ± 0.43%, P < .01), whereas that in the noncytotoxic group (15 patients) was not different from healthy controls (0.14% ± 0.38% vs 0.27% ± 0.43%, P = .23). *P < .01.
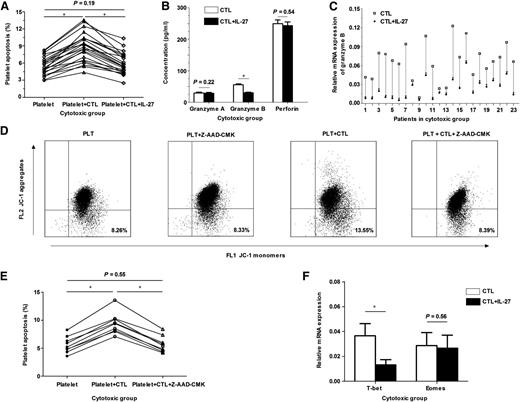
IL-27 has been shown to alleviate autoimmune diseases by decreasing inflammatory factors.10,11,18 In this study, we evaluated the effect of IL-27 on CTL cytotoxicity toward autologous platelets in ITP. In the cytotoxic group, there was no difference in the apoptosis of platelets cultured alone or with IL-27; however, the apoptosis of platelets cultured with CTLs plus IL-27 was significantly lower than that of platelets cultured with CTLs (Figure 2A). When IL-27 was added, the apoptosis of platelets cultured with CTLs returned nearly to the level of the control platelets (spontaneous platelet apoptosis), indicating that IL-27 could significantly inhibit CTL cytotoxicity toward platelets. In the noncytotoxic group and healthy controls with no obviously increased CTL cytotoxicity, IL-27 had no effect on CTLs. These results suggest that in ITP patients with high CTL-mediated platelet apoptosis, IL-27 could significantly alleviate CTL cytotoxicity. Chow et al recently demonstrated in a murine model that infused autoreactive CTL-mediated thrombocytopenia was not sensitive to intravenous γ-globulin, which had effects in antibody-mediated thrombocytopenia.3 In this case, IL-27 might be effective. Several studies have shown that IL-27 has an important role in augmenting the generation of functional CTLs against various tumors,14,19 which differed from our results. This might be mainly due to the pleiotropic effects of IL-27 and the different immunologic conditions between patients with tumors and those with autoimmune diseases.
In the cytotoxic group, IL-27 inhibited CTL-mediated platelet apoptosis by decreasing granzyme B expression. (A) In the cytotoxic group (n = 23), the apoptosis of platelets cultured with CTLs was significantly higher than that of platelets cultured alone (8.85% ± 2.49% vs 5.52% ± 1.58%, P < .01); however, after the addition of IL-27, the apoptosis decreased significantly (5.79% ± 1.86% vs 8.85% ± 2.49%, P < .01) and almost returned to the level of spontaneous platelet apoptosis (5.79% ± 1.86% vs 5.52% ± 1.58%, P = .19). (B) In the cytotoxic group (n = 23), a significant reduction was observed in the concentration of granzyme B in CTLs cultured with IL-27 compared with that without IL-27 (30.54 ± 7.49 pg/mL vs 56.20 ± 12.69 pg/mL, P < .01), but not in granzyme A (28.84 ± 10.03 pg/mL vs 29.91 ± 10.12 pg/mL, P = .22) or perforin (245.80 ± 53.88 pg/mL vs 249.42 ± 56.32 pg/mL, P = .54). (C) In the cytotoxic group (n = 23), the mRNA expression of granzyme B in CTLs decreased after the addition of IL-27 (0.0220 ± 0.0158 vs 0.0655 ± 0.0292, P < .01). (D) The representative dot-plots characterized the apoptosis of platelets cultured alone (8.26%), with Z-AAD-CMK (8.33%), with autologous CTLs (13.55%), or with autologous CTLs plus Z-AAD-CMK (8.39%). (E) Granzyme B inhibition analysis was performed in 9 patients of the cytotoxic group. The apoptosis of platelets cultured with CTLs were significantly higher than that of platelets cultured alone (9.38% ± 1.89% vs 5.59% ± 1.46%, P < .01); however, after the addition of granzyme B inhibitor Z-AAD-CMK, the apoptosis decreased (5.67% ± 1.41% vs 9.38% ± 1.89%, P < .01) and returned to the level of spontaneous platelet apoptosis (5.67% ± 1.41% vs 5.59% ± 1.46%, P = .55). (F) In the cytotoxic group (n = 23), the mRNA expression of T-bet in CTLs was significantly decreased after the addition of IL-27 (0.0133 ± 0.0042 vs 0.0366 ± 0.0098, P < .01), whereas Eomes was unchanged (0.0267 ± 0.0102 vs 0.0287 ± 0.0104, P = .56). *P < .01.
In the cytotoxic group, IL-27 inhibited CTL-mediated platelet apoptosis by decreasing granzyme B expression. (A) In the cytotoxic group (n = 23), the apoptosis of platelets cultured with CTLs was significantly higher than that of platelets cultured alone (8.85% ± 2.49% vs 5.52% ± 1.58%, P < .01); however, after the addition of IL-27, the apoptosis decreased significantly (5.79% ± 1.86% vs 8.85% ± 2.49%, P < .01) and almost returned to the level of spontaneous platelet apoptosis (5.79% ± 1.86% vs 5.52% ± 1.58%, P = .19). (B) In the cytotoxic group (n = 23), a significant reduction was observed in the concentration of granzyme B in CTLs cultured with IL-27 compared with that without IL-27 (30.54 ± 7.49 pg/mL vs 56.20 ± 12.69 pg/mL, P < .01), but not in granzyme A (28.84 ± 10.03 pg/mL vs 29.91 ± 10.12 pg/mL, P = .22) or perforin (245.80 ± 53.88 pg/mL vs 249.42 ± 56.32 pg/mL, P = .54). (C) In the cytotoxic group (n = 23), the mRNA expression of granzyme B in CTLs decreased after the addition of IL-27 (0.0220 ± 0.0158 vs 0.0655 ± 0.0292, P < .01). (D) The representative dot-plots characterized the apoptosis of platelets cultured alone (8.26%), with Z-AAD-CMK (8.33%), with autologous CTLs (13.55%), or with autologous CTLs plus Z-AAD-CMK (8.39%). (E) Granzyme B inhibition analysis was performed in 9 patients of the cytotoxic group. The apoptosis of platelets cultured with CTLs were significantly higher than that of platelets cultured alone (9.38% ± 1.89% vs 5.59% ± 1.46%, P < .01); however, after the addition of granzyme B inhibitor Z-AAD-CMK, the apoptosis decreased (5.67% ± 1.41% vs 9.38% ± 1.89%, P < .01) and returned to the level of spontaneous platelet apoptosis (5.67% ± 1.41% vs 5.59% ± 1.46%, P = .55). (F) In the cytotoxic group (n = 23), the mRNA expression of T-bet in CTLs was significantly decreased after the addition of IL-27 (0.0133 ± 0.0042 vs 0.0366 ± 0.0098, P < .01), whereas Eomes was unchanged (0.0267 ± 0.0102 vs 0.0287 ± 0.0104, P = .56). *P < .01.
Perforin/granzyme-induced apoptosis is the main pathway used by CTLs. Increased granzymes and perforin were found in patients with ITP and diabetes.20,21 In the present study, the supernatant concentrations and messenger RNA (mRNA) expression of granzyme A, granzyme B, and perforin were measured. Consistent with our previous study,16 granzyme B and perforin were increased in the cytotoxic group compared with healthy controls. When IL-27 was added, a significant reduction in granzyme B expression was observed in the cytotoxic group, whereas no difference was observed in granzyme A or perforin (Figure 2B-C). There were no significant differences in granzyme A, granzyme B, or perforin expression in the presence or absence of IL-27 in the noncytotoxic group and healthy controls. To further investigate the role of granzyme B in CTL-mediated platelet apoptosis in ITP, granzyme B inhibition analysis was performed in 14 patients (9 in the cytotoxic group and 5 in the noncytotoxic group; supplemental Table 1) by the application of a granzyme B inhibitor Z-AAD-CMK. Z-AAD-CMK significantly inhibited CTL-mediated platelet apoptosis in the 9 patients of the cytotoxic group (Figure 2D-E). However, there was no difference in the apoptosis of platelets cultured alone or with Z-AAD-CMK (Figure 2D). In addition, Z-AAD-CMK had no effect in the 5 patients of the noncytotoxic group. Our results revealed that CTL-mediated platelet apoptosis in ITP was dependent on granzyme B activity and that the inhibitory effect of IL-27 on CTL cytotoxicity was directly related to the reduction of granzyme B expression.
Because 2 T-box family members, T-bet and Eomes, cooperatively controlled the effector functions of CTLs,22,23 we measured their expression in CTLs. The mRNA expression of T-bet and Eomes in CTLs was increased in the cytotoxic group (23 patients) compared with the noncytotoxic group (15 patients) and healthy controls (12 volunteers) (T-bet, 0.0366 ± 0.0098 vs 0.0123 ± 0.0065 and 0.0110 ± 0.0034, P < .01; Eomes, 0.0287 ± 0.0104 vs 0.0169 ± 0.0066 and 0.0156 ± 0.0058, P < .01, respectively). In the cytotoxic group, the mRNA expression of T-bet was significantly decreased after IL-27 intervention; however, Eomes was unchanged (Figure 2E). This is in line with the fact that T-bet is essential for the development of CTL-dependent autoimmune diabetes,24 and T-bet is required for the induction of perforin and granzyme B in CTLs.25
Taken together, we demonstrated that IL-27 could inhibit CTL cytotoxicity toward autologous platelets in ITP by decreasing granzyme B expression. The novel discovery of the inhibitory effect of IL-27 on CTL activity in ITP may be beneficial for some ITP patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81125002, 81200344, 81270578, 81370623), State Program of National Natural Science Foundation of China for Innovative Research Group (81321061), 973 Program (2011CB503906), and the Independent Innovation Foundation of Shandong University (2012TS134).
Authorship
Contribution: H.Z., J.-h.Q., T.W., X.-g.L., M.H., and J.P. performed research, analyzed data, and wrote the manuscript; Y.-y.Y., X.-n.L., X.L., Y.-w.W., Y.H., and L.-z.L. performed research, analyzed data, and corrected the paper; and all authors read and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jun Peng, Department of Hematology, Qilu Hospital, Shandong University, 107 West Wenhua Rd, Jinan, Shandong 250012, China; e-mail: junpeng88@sina.com.cn; Xin-guang Liu, Cancer Center, Shandong University, 107 West Wenhua Rd, Jinan, Shandong 250012, China; e-mail: liuxingrant@163.com; and Ming Hou, Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education and Chinese Ministry of Health, Qilu Hospital, Shandong University, 107 West Wenhua Rd, Jinan, Shandong 250012, China; e-mail: houming@medmail.com.cn.
References
Author notes
H.Z., J.-h.Q., and T.W. contributed equally to this study.