Key Points
Carfilzomib 56 mg/m2 provided a high overall response rate with a remarkable duration of response in patients with R/RMM.
Nonhematologic grade 3/4 AEs likely related to carfilzomib treatment included hypertension and heart failure.
Abstract
Standard carfilzomib (20 mg/m2 cycle 1, 27 mg/m2 thereafter; 2- to 10-minute infusion) is safe and effective in relapsed or refractory multiple myeloma (R/RMM). We report phase 2 results of carfilzomib 20 mg/m2 on days 1 to 2 of cycle 1, 56 mg/m2 thereafter (30-minute infusion), in R/RMM with the option of adding dexamethasone (20 mg) for suboptimal response/progression. Forty-four patients enrolled, all having prior bortezomib and immunomodulatory drugs and a median of 5 prior regimens. Of 42 response-evaluable patients, 23 (55%) achieved at least partial response (PR). Median (95% confidence interval) duration of response, progression-free, and overall survival were 11.7 (6.7-14.7), 4.1 (2.5-11.8), and 20.3 months (6.4-not estimable), respectively. High-risk cytogenetics did not impact outcomes. Treatment was active in bortezomib-refractory subgroups, but these patients tended to have poorer outcomes. Four/10 patients with prior allogeneic transplant achieved at least PR. Of 6 patients who responded, progressed and had dexamethasone added, 4 achieved at least stable disease. The most frequent grade 3/4 adverse events (AEs) possibly related to carfilzomib included lymphopenia (43%), thrombocytopenia (32%), hypertension (25%), pneumonia (18%), and heart failure (11%). Seven patients (16%) discontinued treatment due to AEs. Carfilzomib 56 mg/m2 ± dexamethasone was tolerable and provided durable responses. This trial was registered at www.clinicaltrials.gov as #NCT01351623.
Introduction
Carfilzomib, a selective proteasome inhibitor, targets and irreversibly binds to the β5 subunit of the constitutive 26S proteasome and LMP7 immunoproteasome, resulting in sustained inhibition of chymotrypsin-like activity and apoptosis of myeloma cells.1-4 The efficacy and safety of single-agent carfilzomib has been demonstrated in a series of phase 2 studies in advanced multiple myeloma (MM), including the pivotal phase 2 trial PX-171-003-A1 in relapsed and refractory disease (N = 266).5-9 Generally in these studies, carfilzomib was administered as a 2- to 10-minute IV infusion twice weekly for 3 weeks of a 4-week cycle at a 20 mg/m2 dose for cycle 1 and 27 mg/m2 thereafter (20/27 mg/m2). In PX-171-003-A1, carfilzomib 20/27 mg/m2 provided an overall response rate (ORR) of 23.7% in 257 response-evaluable patients with median duration of response (DOR) of 7.8 months.5 Grade 3/4 adverse events (AEs) were generally hematologic, and peripheral neuropathy was infrequent with no grade 4 events. Based on these studies, carfilzomib is currently indicated in the United States for patients with relapsed and refractory MM using the 20/27 mg/m2 dose.
As carfilzomib continues to be developed, its use at higher doses to improve depth and DOR in relapsed and refractory settings is being investigated. In preclinical studies, carfilzomib demonstrated minimal off-target activity and minimal neurotoxicity, which may allow for higher doses,10 and longer infusion times improved tolerability by lowering the maximum plasma concentration.11 In the phase 1/2 PX-171-007 study (NCT0053184) in patients with relapsed or refractory (R/R) MM, carfilzomib was administered as a 30-minute IV infusion at doses ranging from 20/36 mg/m2 to 20/70 mg/m2 with a maximum tolerated dose (MTD) of 20/56 mg/m2.12 Efficacy outcomes from preliminary reports suggested improved activity at higher carfilzomib doses with manageable AEs.
Based on the initial findings of the PX-171-007 study,12 we conducted a phase 2 single-center study in patients with relapsed or refractory multiple myeloma (R/RMM) to assess carfilzomib at a dose of 20/56 mg/m2 administered IV over 30 minutes, using the standard twice-weekly dosing schedule. In patients with a suboptimal response (less than partial response [PR]) to single-agent carfilzomib after 2 cycles, low-dose dexamethasone (20 mg) was added prior to each carfilzomib dose to improve activity. This strategy was based on the preclinical findings that suggested synergy between carfilzomib and dexamethasone2 and historical MM studies that showed the benefit of adding dexamethasone to bortezomib.13-16 The objective of the current study was to determine whether the efficacy and safety of carfilzomib 56 mg/m2 ± low-dose dexamethasone in patients with R/RMM would warrant additional studies.
Methods
This was an investigator-initiated, phase 2, single-arm, single-center, open-label study designed and conducted in accordance with the Declaration of Helsinki. The study protocol received institutional review board approval and all patients provided written informed consent.
Patient selection
Patients 18 years of age or older were eligible to enroll if they had measurable symptomatic MM17 that has relapsed or was refractory to at least 2 prior lines of therapy. Prior treatment with bortezomib was required, as was prior treatment with an immunomodulatory drug (IMiD; thalidomide or lenalidomide). Other eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 to 2, adequate hepatic function (serum alanine aminotransferase ≤3.5 times the upper limit of normal and serum direct bilirubin ≤2 mg/dL), as well as an absolute neutrophil count ≥1 × 109/L, hemoglobin ≥8 g/dL, a platelet count ≥50 × 109/L, and a creatinine clearance ≥15 mL per minute. Patients with significant cardiovascular disease (eg, uncontrolled angina or New York Heart Association class III/IV heart failure) were not eligible, nor were patients with grade 3/4 neuropathy.
Treatment plan
Carfilzomib was administered as a 30-minute IV infusion on days 1, 2, 8, 9, 15, and 16 of a 28-day cycle. The initial dose was 20 mg/m2 on days 1 and 2 of cycle 1, which was then escalated to 56 mg/m2 thereafter as tolerated. If patients achieved at least a PR after 2 cycles of treatment, single-agent carfilzomib was continued until progression of disease at which time low-dose dexamethasone (20 mg prior to each carfilzomib dose) could be added to restore antimyeloma activity and prolong treatment. Low-dose dexamethasone could also be added to carfilzomib for patients who had not achieved at least PR after 2 cycles. The combination of carfilzomib and dexamethasone was continued until progression of disease or an intolerable AE. The carfilzomib dose could be held to resolve hematologic and nonhematologic events, and reduced (45, 36, 27, and then to 20 mg/m2) to resolve nonhematologic events with the opportunity to reescalate the dose as indicated. If grade 3/4 nonhematologic toxicities could not be resolved within 3 weeks, treatment was discontinued. After a total of 12 cycles, the dosing frequency of carfilzomib could be reduced to days 1, 2, 15, and 16 of the 28-day cycle.
As part of the treatment protocol, 500 mL of normal saline was administered pre- and postdosing of carfilzomib for cycle 1 along with 8 mg of dexamethasone and 250 mcg of palonosetron. At the discretion of the treating physician, hydration could be reduced to 250 mL of normal saline pre- and postdosing in patients with preexisting cardiovascular or pulmonary risk factors. For cycle 2 and beyond, patients received 250 mL of normal saline prior to carfilzomib dosing and 8 mg of dexamethasone per the treating physician’s discretion along with 250 mcg of palonosetron. Patients received acyclovir 400 mg daily for herpes zoster prophylaxis. Other supportive measures included allopurinol for patients at risk of tumor lysis syndrome.
Study endpoints and assessments
Outcomes were assessed in the per-protocol population (patients who completed 4 cycles of treatment or whose disease progressed prior to completion of 4 cycles), response-evaluable patients (patients who met eligibility criteria and received at least 1 dose of therapy), and the safety population (patients who received any study drug). The primary endpoint of the study was ORR (PR or better) after 4 cycles of treatment were completed in the per-protocol population. Secondary endpoints included DOR, progression-free survival (PFS), overall survival (OS), time to progression, and safety. Response was assessed according to the criteria of the International Myeloma Working Group (IMWG), including the categories of stringent complete response (sCR), complete response (CR), very good partial response (VGPR), PR, stable disease (SD), and progression of disease (POD),18,19 with the addition of minimal response (MR) according to the European Group for Blood and Marrow Transplantation.20 Serum M protein (by electrophoresis and immunofixation) and serum-free light chains were measured at screening and on day 16 of each cycle. Urine M protein was measured at screening and day 1 of cycle 2 and each subsequent cycle. Bone marrow aspirate and biopsy were performed at screening for morphology, immunohistochemistry, flow cytometry, and identification of cytogenetic abnormalities by metaphase cytogenetics and interphase fluorescence in situ hybridization (FISH) studies. High-risk abnormalities included deletion 1p36, 1q25 gain, translocations involving chromosome 14 including t(4;14), t(14;16), and deletion 17p. AEs were assessed according to the Common Terminology Criteria for Adverse Events (version 4.0).21
Statistics
This study used a Simon optimal 2-stage design.22 The treatment was considered of interest if the ORR was 30% or more and of no interest if 10% or less. The probabilities of type I and II errors were 0.10. The first stage of the study enrolled a cohort of 12 patients. If after 4 cycles at least 2 patients (17%) achieved a PR or better, then enrollment was expanded to a total of 35 per-protocol patients. Based on a nonevaluable rate of 15%, planned enrollment was 41 patients. If 6 of 35 per-protocol patients (17%) achieved at least PR after 4 cycles, the treatment was deemed effective and of interest.
Descriptive statistics were used to summarize categorical and continuous data, and Kaplan-Meier methodology was used to summarize time-to-event endpoints, with the log-rank test used to compare survival distributions. The given endpoints were estimated in the response-evaluable population with OS and PFS defined as time from study enrollment to death and progression or death, respectively, and DOR defined as the time from first observation of at least PR to progression or death. Statistical analyses were performed using “R” (version 3.0.1) (http://www.r-project.org/).
Results
Baseline characteristics
Forty-four patients were enrolled from May 17, 2011 to January 9, 2013. The median age was 63 years (range, 45-86 years), 43% were male, and 38% had an ECOG performance status score of 2 (Table 1). High-risk cytogenetic/FISH markers were detected in 45% of patients. This was a heavily pretreated population with a median of 5 (range, 1-11) prior lines of therapy. All patients had prior treatment with bortezomib and an IMiD with 77% refractory to bortezomib and 64% refractory to both bortezomib and lenalidomide. Notably, 72% of patients had prior autologous stem cell transplantation (SCT) and 22% had previously undergone both autologous and allogeneic SCT.
Patient and treatment disposition
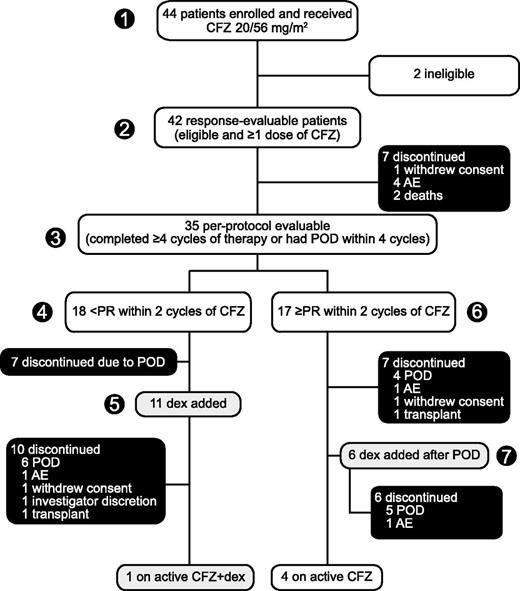
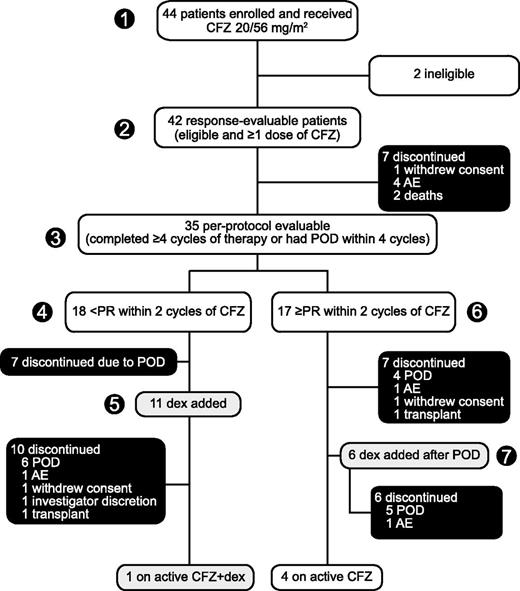
Figure 1 presents patient flow at data cutoff June 27, 2013. All 44 patients received ≥1 dose of carfilzomib and were available for safety evaluation. Two patients were incorrectly enrolled on study with eligibility violations, leaving 42 patients evaluable for response. One ineligible patient did not meet IMWG criteria for R/RMM and the other had a second malignancy in addition to myeloma (of note, the former patient was clearly benefiting from treatment and was continued on study drug). Thirty-five patients met the criteria for the per-protocol population (patients who completed 4 cycles of treatment or whose disease progressed prior to completion of 4 cycles). Seventeen patients in the per-protocol population achieved a PR or better with single-agent carfilzomib; notably, 6 of these patients eventually experienced disease progression and had dexamethasone added to their carfilzomib regimen. The other 18 per-protocol patients achieved less than a PR with single-agent carfilzomib; 11 of these patients continued carfilzomib beyond 2 cycles with the addition of dexamethasone. Overall, 37 of 42 patients eventually discontinued treatment: 22 due to POD (50%), 7 due to AEs (16%), 3 withdrew consent (7%), 2 opted for salvage transplantation (5%), 1 due to investigator discretion (2%), and 2 due to death (5%). Patients received a median cumulative carfilzomib dose of 949 mg/m2 (range, 80-5597 mg/m2) over a median of 4.5 cycles (range, 0.5-21) and a median of 20 doses (range, 3-114).
Patient disposition. Enrollment: May 2011 to January 2013. (1) Forty-four patients enrolled and received single-agent carfilzomib 20/56 mg/m2 with (2) 42 response evaluable and (3) 35 evaluable per protocol. (4) Patients who achieved less than PR within 2 cycles of single-agent carfilzomib could have (5) low-dose dexamethasone (20 mg) added to their regimen and continued treatment until POD or intolerable toxicity. (6) Patients who achieved at least PR within 2 cycles continued single-agent carfilzomib until POD, at which time they had the option of discontinuing treatment or (7) modifying the carfilzomib regimen with the addition of low-dose dexamethasone to potentially overcome resistance and prolong therapy. Data cutoff: June 2013. CFZ, carfilzomib; dex, dexamethasone; CFZ + dex, CFZ in combination with low-dose dex.
Patient disposition. Enrollment: May 2011 to January 2013. (1) Forty-four patients enrolled and received single-agent carfilzomib 20/56 mg/m2 with (2) 42 response evaluable and (3) 35 evaluable per protocol. (4) Patients who achieved less than PR within 2 cycles of single-agent carfilzomib could have (5) low-dose dexamethasone (20 mg) added to their regimen and continued treatment until POD or intolerable toxicity. (6) Patients who achieved at least PR within 2 cycles continued single-agent carfilzomib until POD, at which time they had the option of discontinuing treatment or (7) modifying the carfilzomib regimen with the addition of low-dose dexamethasone to potentially overcome resistance and prolong therapy. Data cutoff: June 2013. CFZ, carfilzomib; dex, dexamethasone; CFZ + dex, CFZ in combination with low-dose dex.
Efficacy
For the primary endpoint, 18 of 35 patients in the per-protocol population achieved a PR or better after 4 cycles of treatment of an ORR of 51% (Table 2). In the response-evaluable population (n = 42), 23 patients achieved a PR or better as their best response over the course of treatment of an ORR of 55%, including 1 CR (2%), 9 VGPR (21%), and 13 PR (31%). Nineteen patients achieved at least PR with single-agent carfilzomib, and 4 of 11 patients who received dexamethasone because of a suboptimal initial response eventually achieved at least PR and continued treatment of an additional 4.5 cycles on average. For 6 patients who achieved at least PR with single-agent carfilzomib, progressed, and then had dexamethasone added to their regimen, 4 achieved at least SD and continued treatment of an additional 5.5 cycles on average.
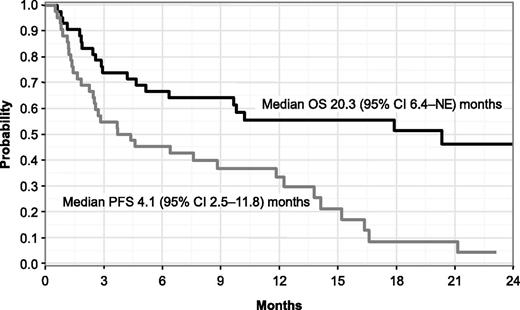
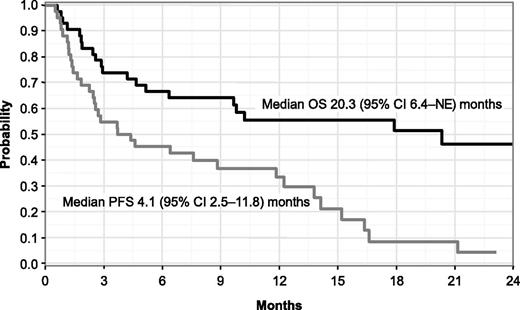
In response-evaluable patients overall, median time to best response was 2 cycles (range, 1-5), and the median DOR was 11.7 months (95% confidence interval [CI], 6.7-14.7). After a median follow-up of 18.4 months (range, 6.5-25.2 months), median PFS was 4.1 months (95% CI, 2.5-11.8) and median OS was 20.3 months (95% CI, 6.4-not estimable) (Figure 2).
PFS and OS by the Kaplan-Meier method in response-evaluable patients (N = 42). NE, not estimable.
PFS and OS by the Kaplan-Meier method in response-evaluable patients (N = 42). NE, not estimable.
Treatment was active across subgroups of interest (Table 3). Although these subgroups are too small for definitive interpretation, there were some trends worth noting. Patients who were refractory to bortezomib had a trend toward a lower ORR compared with patients who were not refractory (47% vs 80%; P = .08), and trends toward a lower PFS rate (1-year estimates 23% vs 70%; P = .18) and a lower OS rate (1-year estimates 43% vs 100%; P = .047), whereas the median DOR was similar (10.5 vs 12.5 months; P = .78). With respect to cytogenetic/FISH risk status, there was no statistically significant difference between patients with high-risk and standard-risk disease with an ORR of 53% vs 59% (P = .76), a 1-year OS rate of 45% vs 67% (P = .09), respectively, and similar DOR and PFS outcomes. In the 10 patients with prior allogeneic transplant, 3 achieved VGPR, 1 a PR, 2 MR, and the disease stabilized in 3 patients.
Safety and tolerability
The most frequent AEs (any grade) considered related (possibly or probably) to carfilzomib 56 mg/m2 included fatigue (48%), nausea (45%), headache (43%), and upper respiratory infection (41%) (Table 4). Grade 3/4 treatment-related AEs were generally hematologic in nature and included lymphopenia (43%), thrombocytopenia (32%), and leukopenia, anemia, and neutropenia (18% for each) (Table 5). Grade 3/4 nonhematologic AEs related to treatment were infrequent with the exception of hypertension (25%), pneumonia (18%), and heart failure (11%). Six patients (14%) had treatment-emergent neuropathy, all grade 1. Two patients (5%) experienced worsening of preexisting neuropathy—from grade 1 to 2 for 1 and from grade 2 to 3 for the other. The addition of dexamethasone to carfilzomib did not have a clinically meaningful impact on the types or rates of AEs. Grade 3/4 AEs attributed to dexamethasone included fatigue, gastric ulcer, and hyperglycemia (1 patient for each).
AEs resulting in discontinuation included decline in left ventricular (LV) systolic function (5 patients), fever (1 patient), and infection and development of myelodysplastic syndrome (1 patient). Dose reductions were required in 17 patients (39%) with the most common reasons being hypertension (5 patients) and fatigue (3 patients). At the time of this analysis, 2 patients had died for reasons other than disease progression. One patient developed pneumonia that progressed to cardiogenic/septic shock, and the other patient died of intracranial hemorrhage. Both of these deaths were considered unrelated to study treatment.
With respect to cardiovascular AEs, some additional patient data are worthy of mention. Of 11 patients who developed grade 3/4 hypertension considered related to carfilzomib, all but 1 had a history of hypertension prior to entering the study. One patient with grade 3/4 hypertension also developed nephrotic-range proteinuria, another developed a thrombotic microangiopathy that improved after holding and then reducing the carfilzomib dose, and 4 patients developed heart failure.
Among 11 patients (25%) who experienced treatment-emergent heart failure of any grade, 4 had prior autologous SCT, 3 had prior autologous and allogeneic SCT, 8 had prior anthracycline treatment, and baseline comorbidities included hypertension (6 patients), hyperlipidemia (5 patients), smoking history (5 patients), and cardiomyopathy (3 patients). Additional details on each of these patients are provided in the supplemental Table (available at the Blood Web site). Five patients suffered respiratory failure and were intubated within the first cycle (2 possibly related to carfilzomib), with 4 of them experiencing a decrease in LV ejection fraction (LVEF). These 5 patients had significant disease burden at baseline, including rapidly progressive disease associated with cytopenias, and died soon after developing heart failure, 1 from cardiogenic/septic shock secondary to pneumonia mentioned earlier and the remaining patients from disease progression within a median of 46 days of their last carfilzomib dose. Four other patients also developed heart failure during cycle 1 (all possibly related to carfilzomib) but with preserved systolic function. These patients were fluid overloaded with no change in their EF, improved after administration of diuretics, and only required brief hospitalization; 2 continued carfilzomib treatment at reduced doses. Whereas the majority of these cardiac events occurred early on, 2 additional patients developed sudden cardiac decompensation after prolonged treatment (12 and 14.7 cycles), presenting as respiratory failure and reduced LVEF (one possibly related to carfilzomib). Both had a history of cardiomyopathy and borderline LVEFs at study entry.
Discussion
In this phase 2, single-arm, single-institution study, carfilzomib 56 mg/m2 provided effective disease control in a population of heavily pretreated patients with R/RMM. More than half of the patients achieved at least a PR and 86% achieved at least SD. Generally, responses were rapid and durable with a median DOR of 11.7 months. Median PFS was shorter at 4.1 months compared with DOR as a number of patients progressed soon after enrolling onto the study, whereas median OS was 20.3 months. Treatment was active across subgroups despite the presence of disease refractory to bortezomib, lenalidomide, or both, although patients who were refractory to bortezomib and lenalidomide tended to have poorer outcomes than those who were not refractory. Importantly, 53% of patients with high-risk cytogenetic/FISH abnormalities achieved at least a PR with a DOR similar to that of patients with standard-risk disease. The addition of dexamethasone to carfilzomib 56 mg/m2 improved response for a number of patients who did not achieve a PR with single-agent carfilzomib within the first few cycles, whereas its addition allowed other patients to reestablish disease control and continue therapy after experiencing disease progression on single-agent carfilzomib.
Although there are limitations to comparisons across studies, it is important to consider the findings reported here in the context of other carfilzomib studies that used various dosing schema in R/RMM populations.5-9 As with our study, the patient population of the pivotal phase 2 PX-171-003-A1 study in R/RMM (N = 266) was heavily pretreated with a median of 5 prior lines of therapy, and nearly all patients had received previous treatment with both bortezomib and an IMiD, 73% were refractory to bortezomib, and 74% had prior SCT. Conversely, only 13% in PX-171-003-A1 had an ECOG performance status of 2. In PX-171-003-A1, carfilzomib 20/27 mg/m2 provided an ORR of 23.7% in 257 response-evaluable patients with a median DOR, PFS, and OS of 7.8, 3.7, and 15.6 months, respectively.5 Patients who were refractory to both bortezomib and lenalidomide had an ORR of 15.4% and a median OS of 11.9 months. Patients with high-risk cytogenetic/FISH markers had a similar ORR compared with standard-risk patients (25.8% vs 24.6%).23
In the absence of randomized trials, and given the size of our study, the true benefit of carfilzomib 56 mg/m2 is uncertain, but treatment efficacy reported here was appreciated in the overall population as well as across the subgroups of interest. The results suggest that carfilzomib 56 mg/m2 ± dexamethasone is highly active in a heavily pretreated population with advanced MM and this regimen may provide added benefit with respect to depth and DOR compared with the currently approved 20/27 mg/m2 dose of single-agent carfilzomib. Importantly, adding low-dose dexamethasone to carfilzomib may allow patients to prolong treatment, which is clinically meaningful in the advanced MM setting where salvage treatment options can be limited. The regimen also appears to overcome, at least partially, high-risk cytogenetic markers overall, but this will require confirmation and the study is too small to assess individual markers (eg, deletion 17p). Although the regimen provided durable responses in subgroups refractory to bortezomib and lenalidomide and at rates that suggest improvement compared with carfilzomib 20/27 mg/m2, there was a trend toward poorer outcomes compared with the corresponding nonrefractory subgroups.
Consistent with our findings, preliminary results from the phase 1/2 study PX-171-007 suggested improved response with carfilzomib 56 mg/m2 as a single agent or in combination with low-dose dexamethasone.12,24 After a dose-escalation phase with single-agent carfilzomib, which determined the MTD to be 20/56 mg/m2, the study was amended to include a cohort of patients treated with carfilzomib 20/45 mg/m2 or 20/56 mg/m2 combined with low-dose dexamethasone (20 mg). The ORR in response-evaluable patients was 60% for single-agent carfilzomib 20/56 mg/m2 (n = 20) and 55% for carfilzomib combined with low-dose dexamethasone (n = 20).
The types of AEs experienced by patients receiving carfilzomib 56 mg/m2 in the current study generally matched those reported with single-agent carfilzomib at standard or lower doses, but with increased rates for some AEs.5-9 In an integrated safety analysis of phase 1/2 studies, which included PX-171-003-A1, safety data were pooled from 526 patients with R/RMM treated with carfilzomib at doses ranging from 15 mg/m2 to 27 mg/m2.25 Rates of grade 3/4 hematologic AEs were lower in the integrated analysis, which reported a rate of (regardless of relation to treatment) 23.4% for thrombocytopenia, 22.4% for anemia, 18.1% for lymphopenia, 10.3% for neutropenia, and 5.3% for leukopenia. The rates (any grade) of fatigue (41.4%), nausea (35%), and diarrhea (22.4%) possibly/probably related to carfilzomib were similar to those reported here, whereas those for upper respiratory tract infection (7.2%) and headache (15.8%) appeared lower. Hypertension (any grade and regardless of its relation to treatment) was reported in 14.3%, with more than half having a history of hypertension. An aggregate of cardiac failure events (congestive heart failure, pulmonary edema, and decreased EF) occurred in 7.2% of patients (5.7% at least grade 3).
In the 007 study, grade 3/4 AEs were generally hematologic in nature as with our study, but the rates for some nonhematologic grade 3/4 AEs appeared to be lower and are worth noting.12,24 The most common nonhematologic grade 3/4 AEs (regardless of causality) in the safety populations of carfilzomib (45 mg/m2 or 56 mg/m2) with low-dose dexamethasone (n = 22) and single-agent carfilzomib 56 mg/m2 (n = 24) were hypertension (9.1% and 13%, respectively) and pneumonia (9.1% and 13%, respectively); the rates for any grade hypertension were 22.7% and 42%, respectively.
In the current study, grade 3/4 hypertension was seen in 25% of patients with 5 patients requiring dose reduction but none discontinuing treatment due to hypertension. The heart failure rate was also higher in the current study (20% for grade 3/4) than has been previously reported. It is possible that the increased rates of cardiovascular events reported here, particularly worsening of previously controlled hypertension, are a dose-dependent phenomenon associated with carfilzomib, but we also cannot rule out the potential impact of established cardiovascular risk factors in our study population. Cardiovascular disease is prevalent in the MM population because of advanced age, disease-related factors, and treatment-related cardiotoxicities across the various treatment agents and modalities, including anthracyclines, alkylating agents, proteasome inhibitors, IMiDs, and allogeneic transplantation.26-31 In the current study population, cardiovascular risk factors were prevalent, including prior hypertension and cardiomyopathy. In addition, prior exposure to therapies associated with cardiotoxicity was common, further confounding the underlying etiology of cardiovascular events. Some patients experiencing cardiovascular events had rapidly progressive myeloma and succumbed to the cancer shortly after experiencing their events. Furthermore, 38% of patients in the safety population in this study had an ECOG performance status of 2 at baseline whereas in the studies included in the integrated analysis it ranged from 5.5% to 20%, indicating a patient population with more advanced disease in this study.25 Finally, clinicians should be aware that overhydration can precipitate heart failure in at-risk patients receiving carfilzomib as may have occurred in 4 patients during this study.
Moving forward, more efficacy and safety data are needed to better characterize the benefit-to-risk profile of carfilzomib 56 mg/m2 ± dexamethasone in relation to standard doses of single-agent carfilzomib across MM populations with respect to response, survival outcomes, and safety. An ongoing randomized phase 2 study in R/RMM will compare low- and high-dose carfilzomib in combination with dexamethasone (NCT01903811). In addition, a head-to-head phase 3 study (ENDEAVOR; NCT01568866) has been initiated and will compare the combination of carfilzomib 20/56 mg2 plus low-dose dexamethasone vs bortezomib (standard dose) plus low-dose dexamethasone in patients with relapsed MM. A substudy of the ENDEAVOR study will include prospective cardiac monitoring in an effort to better understand cardiopulmonary events and the measures that may best mitigate any untoward effect of these drugs on cardiac function. Until data from these studies become available, it would appear practical to ensure adequate blood pressure control before initiating carfilzomib 56 mg/m2 and to closely monitor patients with a history of hypertension or other cardiovascular risk factors. In addition, avoidance of overhydration, especially in patients with cardiac risk factors, and obtaining a baseline echocardiogram prior to initiating therapy may be indicated.
In conclusion, carfilzomib 20/56 mg/m2 ± dexamethasone provided effective and durable disease control in patients with heavily pretreated MM. The data reported here support future studies of carfilzomib 56 mg/m2 either alone or in combination with dexamethasone to validate its potential use as a standard option in clinical practice.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for their participation in this trial. This was an investigator-initiated study that was sponsored by the Memorial Sloan-Kettering Cancer Center and supported by Onyx Pharmaceuticals, Inc., an Amgen subsidiary (South San Francisco, CA). We thank Trixia Camacho (Onyx Pharmaceuticals), for her support and critical review of the manuscript. Medical writing and editorial assistance was provided by Michael Raffin (Fishawack Communications, Inc.), which was supported by Onyx Pharmaceuticals.
Authorship
Contribution: N.L. designed and performed research, collected, analyzed, and interpreted data, and wrote the manuscript; P.H. and S.D. performed statistical analysis; H.L., H.H., A.M.L., G.K., D.J.C., and S.A.G. performed research; I.T. and K.R. collected data; W.L.S. analyzed and interpreted data; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: N.L. served on an advisory board for Onyx Pharmaceuticals. H.L. served on an advisory board for Onyx Pharmaceuticals. S.A.G. has received honoraria from Onyx Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Nikoletta Lendvai, Memorial Sloan-Kettering Cancer Center Myeloma Service, 1275 York Ave, Box 539, New York, NY 10065; e-mail: lendvain@mskcc.org.