Abstract
Although the majority of patients with diffuse large B-cell lymphoma (DLBCL) can be cured with standard rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), patients who fail R-CHOP have a dismal outcome. Thus, optimization of front-line therapy, as well as the development of more effective salvage strategies, remains an important objective. Advances in molecular genetics have vastly improved our understanding of the biological diversity of DLBCL and have led to the discovery of key oncogenic pathways. In addition to the major molecular designations of germinal center B-cell and activated B-cell subtypes, next-generation sequencing technologies have unveiled the remarkable complexity of DLBCL and identified unique molecular targets that may be differentially exploited for therapeutic benefit. These findings have translated into a growing list of promising novel agents. Moving forward, it is of paramount importance to recognize the heterogeneity of DLBCL and to investigate these targeted agents within patient populations who are most likely to benefit. It will be necessary to prioritize drugs that affect key driver pathways and to combine them rationally to optimize their benefit. Improved prognostication and the availability of predictive biomarkers will be crucial to allow for the possibility of individualized risk-adapted therapy.
Introduction
Worldwide, diffuse large B-cell lymphoma (DLBCL) represents the most common subtype of non-Hodgkin lymphoma (NHL), accounting for 30% to 40% of all newly diagnosed cases.1 DLBCL typically presents as an aggressive behaving lymphoma, evolving over months and resulting in symptomatic disease that would imminently be fatal without treatment. Importantly, DLBCL is readily curable with immunochemotherapy in the majority of patients, even in the most advanced cases. The most commonly used initial therapy is rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), but most patients who fail R-CHOP will ultimately die from their disease. Thus, optimization of front-line therapy, as well as the development of more effective salvage strategies, remains an important objective. Within the last decade, advances in molecular genetics have vastly improved our understanding of the biological diversity of DLBCL, leading to the discovery of key oncogenic pathways and novel therapeutic targets. This insight has laid the groundwork for future progress whereby these unique aspects may be selectively exploited, allowing for a more effective and personalized approach to treatment.
Biology of DLBCL
The 2008 version of the World Health Organization (WHO) classification of lymphoid malignancies acknowledges the heterogeneity of DLBCL by recognizing a broad category termed DLBCL, not otherwise specified, as well as a variety of DLBCL subtypes and “other lymphomas of large B cells” that have been identified due to unique clinical and pathologic features.1 Despite this categorization, with the exception of primary central nervous system lymphoma, the majority of patients continue to be treated in a uniform fashion. An ability to measure the biological differences will be crucial to enable individualized therapy in patients who frequently present with morphologically indistinguishable tumors.
Cell of origin
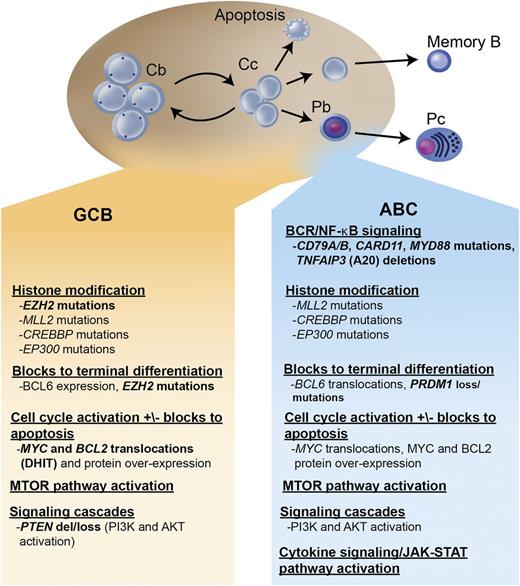
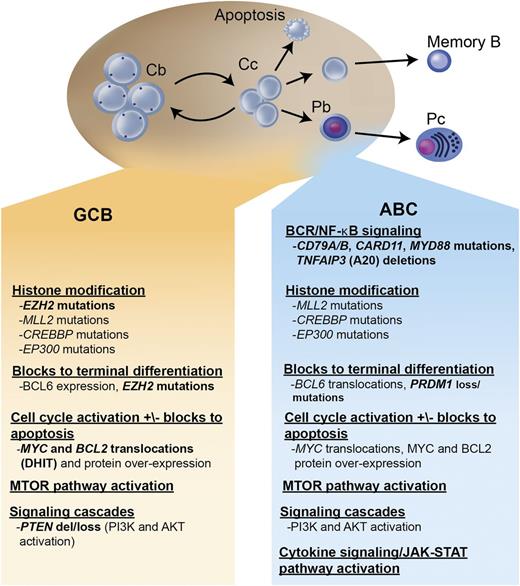
Within the largest category, DLBCL, not otherwise specified, gene expression profiling (GEP) studies have identified ≥2 distinct molecular subtypes, termed germinal center B cell (GCB) and activated B cell (ABC) (with ∼15% of patients remaining unclassifiable), which are believed to represent lymphomas arising from different stages of lymphoid differentiation.2,3 (Figure 1) This molecular distinction has prognostic implications, with the ABC subtype exhibiting an inferior outcome following R-CHOP (3-year progression-free survival [PFS] of ∼40% vs 75%, P < .001).4 More importantly, these molecular subtypes represent lymphomas that are driven by very different intracellular oncogenic signaling pathways that could be differentially exploited for therapeutic benefit.5-9
Key oncogenic pathways in DLBCL. The 2 major molecular subtypes of DLBCL are shown: the GCB and the ABC type. Both tumors arise from stages of differentiation reminiscent of germinal center B cells. The GCB subtype arises from centroblasts, whereas the ABC subtype arises from a plasmablastic cell just prior to germinal center exit. The main oncogenic pathways are listed, as well as the recurrent mutations, gains and losses of genetic material, and characteristic translocations that underlie these pathway perturbations. In bold are oncogenic mechanisms that are preferentially found in 1 molecular subtype. Cb, centroblast; Cc, centrocyte; Pb, plasmablast; Pc, plasma cell; DHIT, double-hit lymphoma; del, deletion; BCR, B-cell receptor.
Key oncogenic pathways in DLBCL. The 2 major molecular subtypes of DLBCL are shown: the GCB and the ABC type. Both tumors arise from stages of differentiation reminiscent of germinal center B cells. The GCB subtype arises from centroblasts, whereas the ABC subtype arises from a plasmablastic cell just prior to germinal center exit. The main oncogenic pathways are listed, as well as the recurrent mutations, gains and losses of genetic material, and characteristic translocations that underlie these pathway perturbations. In bold are oncogenic mechanisms that are preferentially found in 1 molecular subtype. Cb, centroblast; Cc, centrocyte; Pb, plasmablast; Pc, plasma cell; DHIT, double-hit lymphoma; del, deletion; BCR, B-cell receptor.
GCB DLBCL
GCB DLBCLs are believed to derive from lymphoid cells residing in the germinal center and therefore express genes normally detected in germinal center B cells, such as CD10, LMO2, and the transcriptional repressor BCL6.2,3 Approximately 30% to 40% of GCB DLBCLs have a t(14;18) translocation, 30% have c-rel amplification, 20% have mutations of the histone methyltransferase EZH2, and 10% have a deletion of PTEN, all of which are virtually never seen in ABC DLBCL. EZH2 is a master regulator of the GCB phenotype and cooperates with BCL6 to mediate lymphomagenesis in GCB DLBCL.10-12 A recurring somatic point mutation within exon 15 of the EZH2 gene has been identified that results in the replacement of a single tyrosine (Tyr461) within the EZH2 protein, leading to gain of function and the increased methylation of histone 3, which may promote lymphomagenesis by transcriptionally silencing key regulator genes.10,13-15 Both BCL6 and EZH2 may represent important selective targets for GCB DLBCL, and studies of potential agents are underway.16,17
Activation of the phosphatidylinositol 3 kinase (PI3K)/AKT/MTOR signaling pathway is instrumental for cellular growth and metabolism and has been shown to be activated by a variety of mechanisms in a spectrum of B-cell lymphomas, including GCB DLBCL. Although deletion of PTEN, a tumor suppressor gene affecting this pathway, has been noted in 10% of GCB DLBCL, the loss of PTEN expression by immunohistochemistry (IHC) was seen in 55% of GCB DLBCL compared with only 14% of non-GCB cases.18 The resultant constitutive activation of the PI3K/AKT/MTOR pathway in GCB DLBCL lacking PTEN may be amenable to therapy with a variety of inhibitors currently in development.
Overexpression of the antiapoptotic protein BCL2 can be seen in both GCB and ABC DLBCL, although due to very different mechanisms. In GCB DLBCL, overexpression is largely due to the presence of the t(14,18) translocation, whereas in ABC DLBCL, other mechanisms, such as transcriptional upregulation and gene amplification, predominate.19 Following treatment with R-CHOP, the negative prognostic impact of BCL2 overexpression appears to pertain only to GCB DLBCL, possibly due to downregulation of BCL2-mediated nuclear factor κB (NF-κB) expression by rituximab in ABC DLBCL.20 The use of BCL2 inhibitors is a promising therapy for DLBCL,21,22 but based on the above finding, may be especially beneficial in the GCB subtype.
ABC DLBCL
ABC DLBCLs are believed to derive from B cells at a plasmablastic stage, just prior to germinal center exit, and therefore express genes that are frequently expressed in mature plasma cells.2,3 The pathogenetic hallmark of ABC DLBCL is the constitutive activation of the NF-κB signaling pathway, which promotes cell survival, proliferation, and inhibition of apoptosis.5,6 This is largely due to constitutive activation of the CBM signaling complex (formed by CARD11, BCL10, and MALT1), which in normal lymphocytes is only transiently active following antigen stimulation. In ABC DLBCL, the CBM complex can be activated by different genetic aberrations; ∼10% harbor activating mutations of CARD11, whereas in the remaining cases, chronic active B-cell receptor signaling engages the CBM pathway.5,23 Chronic active B-cell receptor signaling is mediated through the B-cell receptor (which in 20% of cases harbors mutations in CD79A or CD79B) and downstream kinases, which include spleen tyrosine kinase (SYK), PI3K, Bruton tyrosine kinase (BTK), and protein kinase C β (PKCβ).5 Recurring mutations in MYD88 have been observed in >30% of ABC DLBCL, resulting in upregulation of both the NF-κB and Janus kinase–signal transducer and activator of transcription pathways.24 Finally, loss of function of TNFAIP3 (A20) occurs through both mutation and deletion, resulting in loss of inhibition of NF-κB signaling.25,26 Numerous agents targeting the oncogenic drivers of ABC DLBCL are under evaluation.8
Determining cell of origin
In view of the prognostic and predictive importance of cell of origin, a reliable method of assessing molecular subtype is required. GEP is not routinely available for patient care due to the lack of a standardized commercially available test and the requirement for fresh-frozen tissue specimens, although paraffin-based methodologies are under development.27 Several IHC-based algorithms have been developed as a practical tool for assigning subtype.28 The Hans algorithm has been the most commonly used and designates patients as GCB vs non-GCB based on the presence of 3 IHC markers (CD10, BCL6, and MUM1).29 In a comparison of several IHC algorithms, the Tally algorithm (based on FOXP1, GCET1, CD10, MUM1, and LMO2) was demonstrated to be the most robust.28 Unfortunately, these IHC algorithms remain an imperfect substitution for GEP, partly due to their inherent oversimplification, the poor reproducibility of IHC,30 and the fact that unclassifiable patients are also dichotomized. Nonetheless, they provide a practical way of designating subtype and may be sufficient for the purpose of achieving population enrichment on clinical trials, but are less reliable for individual patient management. More recently, a 20-gene assay using NanoString technology using paraffin-embedded tissue (Lymph2Cx) has proven to be a promising methodology and is undergoing further validation.31
Molecular complexity
GEP studies of DLBCL have also identified molecular signatures related to the microenvironment that were correlated with outcome.4 A prognostically favorable stromal-1 signature reflects extracellular matrix deposition and infiltration of the tumor by macrophages. The less favorable stromal-2 signature identifies tumors associated with a high level of angiogenesis and a high density of blood vessels. These stromal signatures are independent of cell of origin as they can be seen in both GCB and ABC subtypes. In addition, recurring lesions in genes involved in immune recognition and antigen presenting functions have been recognized, suggesting that escape from immune surveillance plays an important role in the pathogenesis of DLBCL.32 These findings raise the possibility that selectively targeting the microenvironment may offer therapeutic benefit.
Use of high-resolution next-generation sequencing technologies has highlighted the remarkable complexity of DLBCL at the single base pair level.33-36 In addition to translocations and gene amplifications, single nucleotide variants and gene copy number losses contribute to the heterogeneity. One of the most interesting findings from recent genome studies is the high frequency of lesions affecting histone/chromatin modification enzymes, such as CREBBP, EP300, MLL2, and EZH2, which together are detected in >50% of DLBCL cases.13,34,35,37 These alterations likely contribute to lymphomagenesis by epigenetically reprogramming the cells. In a recent study, epigenetic subgroups of DLBCL reflecting variability in DNA methylation were identified and associated with outcome,38 which suggests a role for therapeutic agents that target the epigenome.39
Role of MYC and BCL2
MYC oncogene rearrangement is a hallmark of Burkitt lymphoma and can be identified in ∼10% of patients with DLBCL. Increased expression of MYC protein promotes cellular growth and proliferation. Numerous studies have correlated the presence of a MYC rearrangement with a poorer outcome in DLBCL patients treated with R-CHOP.40,41 However, more recent studies have revealed that the impact of MYC is strongly influenced by BCL2. Concurrent MYC and BCL2 translocation, “double-hit” lymphoma, occurs in ∼5% of cases of DLBCL and represents a treatment-refractory subgroup with a median survival of ∼8 months.42,43 For the most part, these cases reside within the GCB subtype. Importantly, not all patients with double-hit lymphoma may have such a poor outcome, and investigations examining the impact of the partner gene of MYC rearrangement may provide further clarification.
More common than MYC translocation, overexpression of MYC protein due to upregulation by additional mechanisms can be measured by IHC in 25% to 30% of DLBCL patients.43,44 Importantly, the negative prognostic impact of MYC protein expression is observed only in patients who simultaneously overexpress BCL2 protein. Patients with dual expression of MYC and BCL2 protein, commonly referred to as dual-expressers, account for ∼25% of DLBCL cases and have a significantly poorer outcome than patients who express only one or neither protein, with a 5-year PFS in the range of 25% following R-CHOP.43,44 Interestingly, although MYC and BCL2 can play a role in both GCB and ABC subtypes, the dual expression of these proteins appears more common in the ABC subtype and may largely contribute to its inferior survival.45
Based on these findings, patients with DLBCL should be assessed for concurrent MYC and BCL2 deregulation at diagnosis (assessing for both the presence of translocations and protein overexpression), such that alternative therapies can be explored in these poor risk subgroups.
Clinical prognostic factors
The International Prognostic Index (IPI), developed prior to the availability of rituximab, is the primary clinical tool used to predict outcome in patients with DLBCL.46 Using 5 negative prognostic features present at the time of diagnosis (age >60 years, stage III/IV disease, elevated serum lactate dehydrogenase level, Eastern Cooperative Oncology Group performance status ≥2, and >1 extranodal site of disease), the IPI segregated patients into 4 outcome groups with 5-year overall survival (OS) ranging from 26% to 73%. The IPI has been reevaluated in patients treated with rituximab-based therapy and has been shown to retain its prognostic utility.47,48 However, the IPI has minimal capacity to identify high-risk patients, because all prognostic categories have a ≥50% chance of cure when treated with R-CHOP. Recently, an enhanced IPI using data from the National Comprehensive Cancer Network (NCCN-IPI) and validated within a population of patients treated in British Columbia has been proposed.49 The NCCN-IPI incorporates the same 5 variables, although further refines the categorization of age and normalized lactate dehydrogenase and identifies the presence of extranodal involvement in the bone marrow, central nervous system, liver/gastrointestinal tract, or lung as a negative parameter rather than the number of extranodal sites. Compared with the IPI, the NCCN-IPI was better able to discriminate a high-risk group with 5-year OS of 33%, although it represented only 8% to 14% of the patients.
Additional clinical factors have been identified that may also contribute to a poorer outcome following rituximab-based therapy. These include maximum tumor diameter ≥10 cm,50 male gender,51 bone marrow involvement with large cell lymphoma but not discordant small cells,52 elevated serum free light chains,53 low absolute lymphocyte/monocyte count,54-56 and vitamin D deficiency.57,58 Increased body mass index has been reported to be a favorable prognostic factor; however, this has not been confirmed in all studies.59,60 Although clinical factors remain surrogates for the underlying biological differences between patients, to date the IPI has not been made redundant by any biological marker. Incorporation of additional clinical factors within future prognostic indices may allow for wider differentiation of outcome.
Front-line therapy
Advanced stage disease
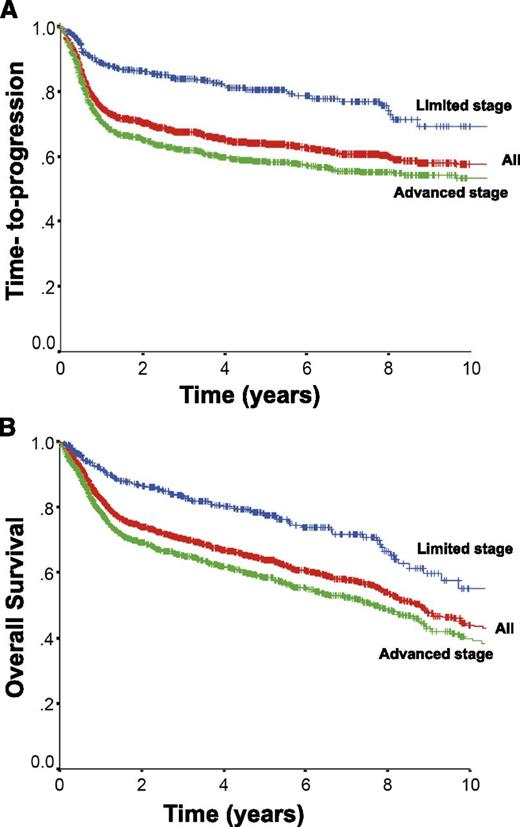
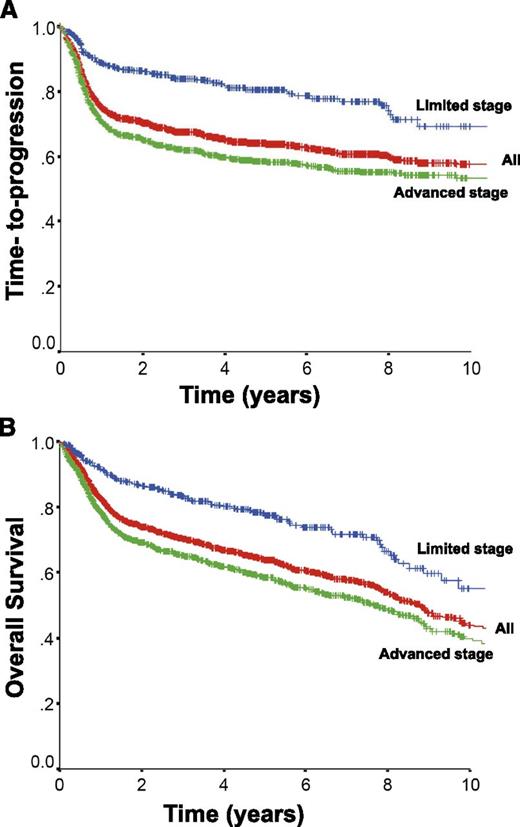
Approximately 75% of patients with DLBCL present with advanced stage disease, commonly defined as Ann Arbor stages 3 and 4 or stages 1 and 2 with associated B-symptoms or bulky disease ≥ 10 cm. The CHOP chemotherapy regimen has been the mainstay of therapy, because more intensive combinations failed to show additional benefit.61 The addition of the anti-CD20 monoclonal antibody rituximab to this chemotherapy backbone dramatically improved outcomes, resulting in a 16% absolute improvement in 10-year OS in the initial trial in elderly patients ≥ 60 years of age.62,63 Additional trials further demonstrated the benefit of rituximab, establishing R-CHOP as the standard of care.64-67 The rituximab with CHOP over age 60 (RICOVER-60) trial also confirmed that 6 cycles of therapy was sufficient for patients with advanced stage disease.65 The time-to-progression and OS for all patients with DLBCL (n = 1660) treated with R-CHOP with curative intent in the province of British Columbia between 2001 and 2013 according to stage of disease is depicted in Figure 2 (unpublished data using the British Columbia Cancer Agency Lymphoid Cancer Database). Approximately 60% of patients will be cured, with more favorable outcomes seen in patients with limited stage disease. Patients who remain event-free at 24 months have recently been shown to have an OS comparable to the general population, further highlighting the importance of optimizing front-line therapy.68
Outcome for all patients with DLBCL treated with R-CHOP in British Columbia between 2001 and 2013 and according to stage at diagnosis. All, n = 1660; limited stage, n = 433; advanced stage, n = 1227.
Outcome for all patients with DLBCL treated with R-CHOP in British Columbia between 2001 and 2013 and according to stage at diagnosis. All, n = 1660; limited stage, n = 433; advanced stage, n = 1227.
Various alternative regimens have been explored in an attempt to improve on R-CHOP, with only 1 study demonstrating an OS benefit (Table 1). A dose intensive regimen of rituximab, doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (R-ACVBP) was associated with a better OS compared with R-CHOP (3-year OS, 92% vs 84%, P = .007) in a randomized phase 3 trial in a select population of patients <60 years of age with only 1 IPI risk factor.69 Despite this demonstrated benefit, R-ACVBP has not become highly used, partly because its value in a general population of patients with DLBCL remains unclear and due to concern for both acute and delayed toxicity.69,70 R-CHOP-14 (R-CHOP administered every 2 weeks) demonstrated no additional benefit, but a higher frequency of toxicity.71,72 A retrospective population-based study suggested a survival benefit with the addition of etoposide to R-CHOP (R-CHOEP) for young high-risk patients; however, this was not observed in a subgroup analysis of a phase 3 trial in young good-risk patients.73,74 The infusional regimen of dose-adjusted etoposide, cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab (DA-EPOCH-R) has yielded promising results in a phase 2 trial in DLBCL, especially within the GCB subtype.75,76 However, confirmation of its merit must await results of a completed phase 3 trial comparing it with R-CHOP.
The value of consolidative autologous stem cell transplantation (ASCT) in the initial management of DLBCL remains a controversial issue, with historical trials yielding conflicting results.77 Several studies evaluating its role following response to rituximab-based chemotherapy have recently been performed. (Table 1) Preliminary results of a randomized trial comparing R-CHOP-14 vs ASCT in young patients with DLBCL demonstrated no difference in outcome.78 In a separate trial, conventional dose R-CHOEP-14 demonstrated a trend toward better outcomes compared with a high-dose therapy approach (R-Mega-CHOEP) for young high-risk patients with aggressive B-cell lymphoma.79 An additional trial with a 2 × 2 factorial design evaluated rituximab dose-dense chemotherapy (R-CHOP-14 or R-Mega-CHOP-14) with or without the addition of ASCT in high-risk patients with DLBCL.80 Although an improvement in PFS was reported with ASCT, no difference in OS was seen. Finally, a US/Canadian Intergroup phase 3 study evaluated the benefit of ASCT in patients with high-intermediate and high-risk aggressive NHL following response to CHOP (with rituximab in only 47% of patients).81 The overall trial results suggested an improvement in PFS but not OS with ASCT. However, an exploratory subgroup analysis demonstrated that the benefit was confined to those with a high-risk IPI score only, where both an improved PFS and OS were seen. In view of the benefit of ASCT in the salvage setting, it is conceivable that a subgroup of patients may benefit from its use upfront. However, the ability to identify this subgroup based on clinical or biological indicators is currently limited, and therefore the use of consolidative ASCT cannot be routinely recommended.
At the present time, deviation away from R-CHOP outside of a clinical trial is difficult to justify because a better therapeutic alternative, without substantial additional toxicity, has not been identified. However, due to the extremely dismal outcome in patients with concurrent translocations of MYC and BCL2 (double-hit), alternate strategies should be explored in this subgroup.42,43 However, it remains unclear which alternate approach will prove beneficial. Several retrospective reviews have suggested that an intensive Burkitt-type regimen or an infusional strategy such as DA-EPOCH-R, followed by stem cell transplantation, may offer benefit.82-84 Similarly, a retrospective review of DLBCL patients treated with DA-EPOCH-R suggested that it may ameliorate the negative prognostic impact associated with the dual expression of MYC and BCL2 by IHC.85 However, patients who are dual-expressers would be more appropriately treated within the context of clinical trials, as their outcome is not as poor as double-hit patients, and measurement of MYC by IHC requires further standardization.
Consolidative radiation therapy and role of PET scanning
In advanced stage patients, consolidative radiation therapy following completion of chemotherapy has been frequently used in an attempt to eradicate potential residual disease from high-risk sites, such as areas of initial bulk or residual masses on computed tomography (CT) scan. Although historical comparative trials suggested a possible benefit,86-88 recent improvement in systemic therapy may have diminished its role. To date, there have been no randomized trials evaluating the role of consolidative radiation therapy in DLBCL in the rituximab era. Several retrospective analyses have suggested a benefit for radiation therapy following R-CHOP, with improved local control and possible overall outcome.90-92 In a recent comparison of patients sequentially treated on the RICOVER-60 trial, the addition of consolidative radiation to sites of initial bulk appeared to improve event-free survival (EFS), with a trend to improved OS.93 Importantly, restaging 18-fluorodeoxyglucose positron emission tomography (PET) scanning was not routinely performed in these studies.
Based on its higher sensitivity compared with CT imaging,94,95 PET scanning (or integrated PET/CT, which is now most commonly used) is recommended for routine staging and response assessment of DLBCL.96,134,135 The introduction of PET has resulted in a more accurate assessment of response and can distinguish persistent disease from residual scar tissue.97 In 2005, the British Columbia Cancer Agency adopted a PET-guided treatment algorithm whereby responding patients with residual masses that remained PET positive following R-CHOP received consolidative radiation when feasible, whereas patients with a negative PET scan were observed. Patients with PET-positive sites amenable to radiation had a favorable outcome comparable to patients who were PET negative, suggesting a possible benefit of the selective use of consolidative radiation therapy and demonstrating that the majority of patients who were PET negative could be spared unnecessary radiation.98 Ongoing randomized studies will further assess the feasibility of a PET-guided approach for patients with advanced stage DLBCL in an attempt to avoid the indiscriminate use of radiation and minimize late toxicities. A full discussion on the use of interim PET scans in DLBCL is beyond the scope of this article. Based on discrepancies in the literature and the need for further standardization of the performance and interpretation of PET scanning in this setting, changing treatment solely on the basis of an interim PET scan is not recommended outside of a clinical trial.135
Limited stage disease
Almost one quarter of patients present with localized disease that can be encompassed within a radiation field and may be amenable to a combined modality treatment approach. These patients typically present with low-risk clinical features and have a more favorable prognosis, although it remains unclear whether this represents a true biological difference or simply a reflection of lesser disease burden. Interestingly, a pattern of delayed relapse has been noted in limited stage patients, which has fueled this speculation99 (Figure 2). To date, only a few trials have explored the optimal treatment strategy for limited stage patients, all of which were performed prior to the introduction of rituximab.100-103 The most influential trial was performed by the Southwest Oncology Group, in which patients with stage I and II intermediate-high grade lymphoma were treated with 8 cycles of CHOP or 3 cycles of CHOP and involved field radiation therapy (IFRT).102 At the time of initial publication, the combined modality approach was associated with a higher PFS and OS and had a better toxicity profile. As a consequence, 3 cycles of CHOP and IFRT were widely adopted as the standard of care for limited stage DLBCL. However, in an updated analysis, the improvement seen with abbreviated chemotherapy and IFRT disappeared at 7 to 9 years of follow-up due to a higher number of late relapses and deaths from lymphoma in that cohort, the majority of which occurred outside of the radiation field.99
Although the use of IFRT may provide a benefit for some patients (particularly in terms of local control), a long-term survival advantage has not been confirmed, and its benefit must be weighed against the potential for delayed toxicity. As more effective chemotherapy is introduced, such as with the introduction of R-CHOP, the marginal benefit of radiation may be diminished. Ultimately, an individualized approach to therapy in limited stage disease seems most rational. The use of IFRT may be preferable in settings in which poor tolerance to chemotherapy is anticipated or when toxicity of IFRT may be minimal due to location of disease, as this would allow the use of abbreviated chemotherapy. Additionally, the selective use of IFRT in patients less responsive to chemotherapy may be considered. A PET-guided treatment algorithm was adopted at the British Columbia Cancer Agency in 2005, whereby patients who remained PET positive after 3 cycles of R-CHOP completed therapy with localized radiation, and patients who were PET negative received 1 final cycle of chemotherapy. Preliminary review of this experience suggests that chemosensitive patients with a negative interim PET scan can be effectively treated with abbreviated systemic therapy (4 cycles of R-CHOP) and avoid the use of radiation.104,105 In patients who do require radiation, a retrospective review suggests that the use of involved nodal radiation therapy appears equivalent to IFRT and may reduce the risk of long-term complications.106 In the future, a personalized treatment approach that relies more on biological characteristics rather than stage of disease will likely be used.
Salvage therapy
Although outcomes in DLBCL have improved with the addition of rituximab, ∼10% to 15% of patients exhibit primary refractory disease (nonresponse or relapse within 3 months of therapy) and an additional 20% to 25% relapse following initial response to therapy. Most relapses occur within the first 2 years; however ∼10% of all progressions occur >5 years after treatment.63 High-dose chemotherapy and ASCT have historically been shown to provide the best chance of cure for patients with chemotherapy-sensitive relapse.107 However, due to advanced age and comorbidities, only half of all patients are eligible for such an intensive approach, and of these transplant-eligible patients, only half will prove to be chemosensitive to salvage therapy and proceed to transplant, of which less than half will be cured. As a consequence, relatively few patients are cured in the salvage setting, as demonstrated by the minimal difference seen in the time-to-progression and OS curves for patients with DLBCL treated in British Columbia (Figure 2).
Several salvage regimens have been developed using drugs that are non-cross-resistant with CHOP, with the aim to reduce tumor burden and confirm chemotherapy sensitivity prior to ASCT. Two recently performed randomized trials have compared the efficacy of the most commonly used regimens108,109 (Table 2). The collaborative trial in relapsed aggressive lymphoma (CORAL) compared R-DHAP (rituximab, dexamethasone, Ara-C, cisplatin) and R-ICE (rituximab, ifosfamide, carboplatin, etoposide) in relapsed/refractory patients with DLBCL prior to ASCT, followed by maintenance rituximab vs observation.109 Overall, the response rates, EFS and OS were similar between the 2 salvage regimens. Prior treatment with rituximab, early relapse within 12 months of diagnosis, and higher IPI score were associated with a poorer outcome. Interestingly, a correlative biomarker analysis suggested that patients with GCB subtype may preferentially benefit from R-DHAP,110 whereas patients with a MYC translocation had a poorer outcome than patients without a MYC translocation, regardless of salvage regimen used.111
In a similarly designed salvage trial performed by the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG), GDP (gemcitabine, dexamethasone, and cisplatin) was compared with DHAP in transplant-eligible patients with aggressive lymphoma (including transformed and T-cell lymphoma).108 Most patients with B-cell lymphoma also received rituximab. With similar response rates, EFS, and OS, the efficacy of (R)-GDP was found to be noninferior to (R)-DHAP and was associated with a more favorable toxicity profile and better quality of life. Based on the above comparisons, the 3 most commonly used salvage regimens appear to be equivalent, although R-GDP has the advantage of the ease of outpatient administration. Finally, both the CORAL and NCIC-CTG trials did not demonstrate a benefit for the use of maintenance rituximab after ASCT.112,113
Patients with primary refractory disease following R-CHOP present the greatest challenge. A review of patients with primary refractory DLBCL treated at the British Columbia Cancer Agency revealed that <10% of patients will achieve durable remissions with secondary therapies.114 Thus, consideration of investigational agents should be foremost in this setting. Similarly, in patients who are ineligible for transplantation, treatment becomes largely palliative, making novel approaches desirable. Outside of a clinical trial, the use of sequential single agent chemotherapy or local radiation therapy for focal symptoms may offer benefit while minimizing treatment-related side effects. The combination of rituximab, gemcitabine, and oxaliplatin (R-GemOx) has been evaluated in transplant-ineligible patients with relapsed or refractory DLBCL and was associated with a favorable overall response rate of 61% and a complete response rate of 44%, with a reasonable toxicity profile.115 Recently, bendamustine combined with rituximab has demonstrated modest efficacy with an overall response rate of 59% and a median PFS of 7 months.116
Novel therapies
Greater insight into the biological heterogeneity of DLBCL has uncovered unique therapeutic targets that have translated into the development of novel agents with the potential for greater tumor specificity and lower generalized toxicity. These agents may ultimately improve outcome when combined with initial therapy, provide an alternative for patients who are too frail for R-CHOP, or provide an effective option in the relapsed/refractory setting. However, in view of the selective activity of many of these novel therapies, it will be vital to evaluate them rationally in patient populations enriched for those who are most likely to benefit. A review of all of the exciting agents in development is beyond the scope of this paper. Examples of some of the prototypic drugs in development, along with their postulated target and the DLBCL subgroup that may preferentially benefit, is listed in Table 3.
Multiple therapies targeting the NF-κB pathway or the B-cell receptor signaling pathway are under evaluation and hold the most promise for ABC DLBCL, where these pathways are constitutively active. Bortezomib, a proteasome inhibitor that blocks the degradation of IκBα (an inactivating protein for NF-κB), has demonstrated selective benefit within the ABC subtype when combined with DA-EPOCH-R in patients with relapsed DLBCL.117 Ongoing trials are evaluating the addition of bortezomib to R-CHOP in untreated DLBCL. Drugs targeting various components of the B-cell receptor cascade (including BTK, SYK, PKCβ, and PI3K) are under evaluation and raise the possibility of targeting multiple components at once. Ibrutinib, an orally available inhibitor of BTK, induced responses in 41% of patients with relapsed ABC DLBCL, but only 5% of patients with GCB DLBCL.118 An ongoing phase 3 trial will evaluate the addition of ibrutinib to R-CHOP in non-GCB DLBCL. Fostamatinib disodium, an orally available SYK inhibitor, has demonstrated clinical responses in >20% of patients with refractory DLBCL in a phase 1/2 trial.119,120 Enzastaurin is an inhibitor of PKCβ and has demonstrated activity in DLBCL,136,137 although a phase 3 trial evaluating enzastaurin as maintenance therapy following R-CHOP in high-risk DLBCL failed to show benefit.121 Finally, idelalisib, an oral selective inhibitor of PI3K-δ, has shown promise in indolent NHL and CLL, but has not been fully evaluated in DLBCL.122,123 Although PI3K is an important component of the B-cell receptor pathway, based on the frequent activation of the PI3K/AKT/MTOR pathway seen in GCB DLBCL, this drug may be more beneficial in that subtype.18
Both ABC and GCB DLBCL frequently overexpress BCL2, which has been shown to negatively impact outcome in both the GCB subgroup and the dual expressers of MYC and BCL2.20,43,44 ABT-199 is a selective inhibitor of BCL2 with a more favorable toxicity profile than its predecessors.22 Responses were seen in 38% of patients with relapsed DLBCL in a phase 1 study, and combination trials are now underway.21
Given the importance of the microenvironment in DLBCL, agents that target this component may have therapeutic benefit. Lenalidomide is an immunomodulatory agent with pleiotropic activity, including antiangiogenic properties and direct tumor cell effects through inhibition of NF-κB.124,125 Studies have demonstrated an overall response rate of ∼30% in relapsed/refractory aggressive lymphoma.126 In a retrospective study of patients with relapsed/refractory DLBCL, lenalidomide appeared to be preferentially active in non-GCB DLBCL, with a higher response seen compared with GCB DLBCL (52% vs 9%, P = .006).127 Lenalidomide has been safely combined with R-CHOP with promising efficacy and may overcome the negative prognostic impact of non-GCB DLBCL.128-130 Several phase 3 studies investigating its role in untreated DLBCL are ongoing, both in combination with R-CHOP and as maintenance therapy.
The addition of rituximab to CHOP has led to the largest improvement in outcome in DLBCL since the introduction of anthracycline-based therapy. Therefore, evaluating agents that may further improve the therapeutic benefit of targeting CD20 is rational. Obinutuzumab is a novel glycoengineered type 2 anti-CD20 monoclonal antibody that has been demonstrated to be more effective than rituximab when combined with chlorambucil in patients with CLL and comorbidities.131 A phase 3 trial comparing obinutuzumab to rituximab in combination with CHOP for DLBCL has been completed and is awaiting evaluation. Additional novel monoclonal antibodies targeting various B-cell markers are also under development.89 Finally, multiple antibody drug conjugates targeting B-cell surface markers are being evaluated.132 As an example, polatuzumab vedotin, an antibody drug conjugate targeting CD79B that is universally expressed in DLBCL, has induced response rates in 48% of patients with relapsed/refractory DLBCL in a phase 2 trial and is undergoing evaluation in combination trials.133
Despite this plethora of emerging therapies, the practical challenges of making further progress in DLBCL remain formidable. Given the relatively favorable outcome with R-CHOP, novel therapies will likely need to be added to this backbone, as in the majority of patients it would not be appropriate to forego curative therapy for an investigational agent. Large patient trials will be required to demonstrate additional benefit, highlighting the need to enrich study populations with those who are most likely to benefit. The identification of predictive biomarkers and development of molecular assays that provide accurate and reproducible measurement in a timely fashion should be undertaken early within the course of drug development, such that a standardized companion diagnostic suitable for routine clinical use can be validated within the context of phase 3 trials. The requirement for appropriate tissue samples and the need to prescreen patients with specialized tests within centralized laboratories will add complexity to the enrollment process and limit the number of patients eligible for inclusion. The resultant delay in treatment initiation will lead to an inevitable selection bias in favor of patients with less aggressive disease. These logistical challenges will need to be taken into consideration when designing biomarker-driven clinical trials to ensure that conduct remains feasible and may require compromise, such as commencing a novel agent following initiation of standard therapy or discontinuing its use when a biomarker is determined to be absent. In view of limited resources and patients available for trial participation, candidate drugs will need to be prioritized based on sound preclinical and early clinical studies with attempt to identify those affecting key driver pathways. As study inclusion shifts toward smaller subsets of patients with DLBCL, wider collaborative efforts will be required to ensure success.
Conclusions
Outcome in DLBCL has improved dramatically over the last decade with the addition of rituximab to CHOP, which remains the current standard of care. However, patients who fail R-CHOP continue to have a dismal outcome, highlighting the limits of standard cytotoxic therapy in the setting of chemoresistant disease. To optimize future management, it will be imperative to acknowledge the molecular heterogeneity of DLBCL and to investigate novel targeted agents within the biological subsets that will most likely benefit. In addition to the designation by cell of origin, a more detailed understanding of the genetic complexity of individual tumors will be required to rationally select the best combination of agents. Currently, patients with double-hit lymphoma, as well as the dual expressers of MYC and BCL2, represent poor-risk subsets in which alternative strategies should be explored. Improved prognostication and the availability of predictive biomarkers will be crucial to allow for the possibility of individualized risk-adapted therapy.
Acknowledgments
The authors thank Dr Barbara Meissner and Dr David Scott for help preparing Figure 1.
R.D.G. is supported by a Terry Fox Research Institute Program Project Grant (1023).
Authorship
Contribution: L.H.S. and R.D.G. wrote the paper.
Conflict-of-interest disclosure: L.H.S. received honoraria or consulted for Roche/Genentech, Celgene, Janssen Pharmaceuticals, Gilead, and Lundbeck. R.D.G. received honoraria or consulted for Roche/Genentech, Celgene, Janssen Pharmaceuticals, and Gilead.
Correspondence: Laurie H. Sehn, British Columbia Cancer Agency and the University of British Columbia, 600 W 10th Ave, Vancouver, BC V5Z 4E6, Canada; e-mail: lsehn@bccancer.bc.ca.