Abstract
Mantle cell lymphoma (MCL) is a rare and aggressive form of non-Hodgkin’s lymphoma that generally affects older individuals and continues to have one of the worst outcomes of all the lymphomas. Over the last decade, there has been a widespread adoption of cytarabine-based therapy in younger patients, and the incorporation of rituximab into chemotherapeutic regimens has become an evidence-based standard of care. However MCL remains a largely incurable disease, and following relapse, it can be a challenge to manage. Although it is possible to define prognosis reliably, there are, as yet, no clear diagnostic or response-adjusted parameters that can help to guide therapeutic decisions. However, there are a number of highly active targeted therapies that are moving into the clinic that are set to transform the therapeutic paradigm for this disease in the very near future. This review will explore the molecular pathogenesis of MCL and the current and evolving therapeutic strategies for this disease.
Molecular pathogenesis
Mantle cell lymphoma (MCL) is genetically characterized by the translocation t(11;14)(q13;q32) and the overexpression of CCND1 that probably facilitates the transformation of the cells by deregulating the cell cycle. This initial event is acquired in pre-B cells of the bone marrow and seems to be followed by 2 different molecular pathways that configure 2 clinical and biological subtypes of the disease.1,2 The classical and most common form of MCL derive from mature B cells that do not enter the follicular germinal center and carry no or a limited number of IGHV somatic mutations. These tumors express the transcription factor SOX11, are genetically unstable, and tend to accumulate alterations in cell cycle regulatory genes, the DNA damage response pathway, and cell survival mechanisms. The acquisition of these alterations results in a more aggressive behavior. The second less common subtype of MCL is characterized by cells that also carry the t(11,14) and CCND1 overexpression but have experienced the follicular germinal center and carry IGHV with somatic hypermutations. These cells are genetically stable, SOX11 expression is negative or very low, and the tumor tends to disseminate to the peripheral blood and spleen more than to the lymph nodes. The disease seems to be stable and asymptomatic for long periods of time, but some tumors may acquire additional alterations in genes such as TP53 that lead to the progression of the disease and transformation to a more aggressive variant.1,3,4
The relevance of SOX11 in the pathogenesis of MCLs is highlighted by its negative expression in all mature lymphoid cells and virtually all mature B-cell neoplasms.5,6 SOX11 promotes tumor growth of MCL cells in vivo and regulates a broad transcriptional program that includes B-cell differentiation, cell proliferation, apoptosis, and angiogenesis among other oncogenic mechanisms.7 One of the strongest direct targets of SOX11 is PAX5, a master regulator of B-cell differentiation that is physiologically downregulated in the terminal steps toward plasma cells. The forced expression of PAX5 by SOX11 may prevent the cells from responding to normal differentiation signals, blocking their maturation process.7 A subset of MCL does not carry the t(11,14) translocation and CCND1 expression but has the same pathological and clinical characteristics of MCLs with this genetic alteration. Intriguingly, 55% of these tumors carry CCND2 translocations and all of them have SOX11 expression, emphasizing the relevance of this transcription factor in the pathogenesis of MCLs.8
The most common alterations further deregulating cell cycle in MCLs involve the INK4a/CDK4/RB1 and ARF/MDM2/TP53 pathways.1 The CDKN2A locus (9p21), frequently deleted in MCL, connects both pathways encoding the CDK inhibitor INK4a and the positive p53 regulator ARF. TP53 is commonly mutated gene in MCL (19-28%), and RB1 is also inactivated by point mutations or gene deletions in occasional cases. Gene amplification leads to the overexpression of CDK4, MDM2, and BMI1, which in turn repress the CDKN2A locus. The relevance of cell cycle deregulation in MCL is highlighted by the poor prognosis conferred by high proliferative activity measured either by a gene expression signature or the Ki67 index.9
Recent genome-wide studies using next-generation sequencing (NGS) have expanded the perspective of genes and pathways involved in the development of MCLs.10,11 These studies have confirmed that the most common secondary alteration in MCLs is the mutation of the DNA damage sensor ATM (42-55% of cases), usually associated with 11q deletions and a high number of chromosomal alterations. These alterations seem to accumulate in tumors expressing SOX11.10 Novel mechanisms identified include activating mutations in NOTCH1/2 in ∼10% of the tumors associated with an aggressive evolution.10,12 Mutations in several chromatin modifiers such as WHSK1 (10%), MLL2 (14%), and MEF2B (3%) have also been detected almost exclusively in MCLs expressing SOX11. WHSK1 mutations seem to deregulate a set of genes enriched in proliferation and cell cycle control similar to the signature seen in plasma cell myeloma with the t(4,14) involving the same gene.10
Somatic mutations in regulatory genes of the nuclear factor κB (NF-κB) pathway have been identified in ∼10% to 15% of MCLs.10,13 BIRC3 is the most commonly affected gene (6-10%), and the mutations are preferentially truncating. Other alterations in both canonical and alternative NF-κB pathways include recurrent inactivating mutations of TRAF2, activating mutations of TLR2, and occasional mutations in CARD11, MAP3K14 (NIK), and IKBKB (IKK-β) suggesting that the NF-κB pathway may be activated by genetic alterations in a higher number of cases than initially thought. A practical consequence of these alterations is their possible relationship with resistance of MCL cells to inhibitors of the B-cell receptor (BCR) pathway as demonstrated in MCL cell lines.13,14
In addition to frequent genetic alterations, MCL have deregulation of different signaling pathways that may be important targets of new drugs.1,15 The promising results obtained with the inhibitors of the BCR signaling suggest that survival of MCL cells depend on the activation of this pathway, although the mechanisms are not entirely understood.16 Activation of the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway has been observed in MCLs and is an effective target for new therapies.1 Recent studies are emphasizing the potential relevance of the interactions between MCL cells and the microenvironment. In this sense, activating mutations of TLR2 have been identified in occasional SOX11-negative MCLs, and these mutations trigger secretion of high levels of interleukin 6 (IL6) and IL1RA.10 Conversely, IL6 and IL10 may activate the signal transducer and activator of transcription 3 pathway, particularly in MCL carrying IGHV hypermutations, suggesting that this subtype of MCL may be dependent on microenvironment signals.17
Current standard therapy
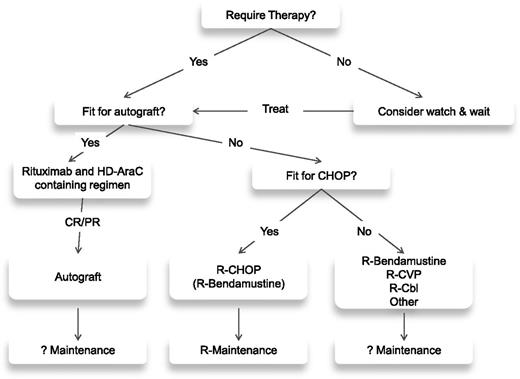
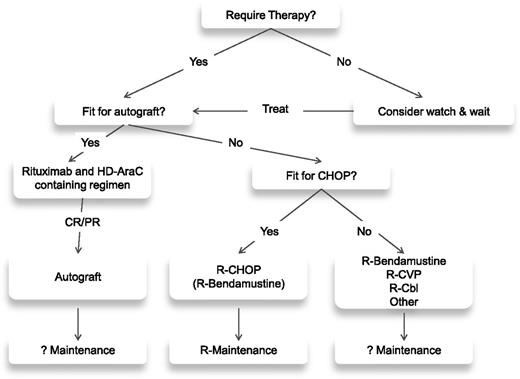
The initial therapeutic decision for a patient with MCL is dictated by the age and, more importantly, the fitness of the patient (see treatment algorithm; Figure 1). For the fitter patients, the treatment of choice involves a cytarabine-based regimen, which is usually consolidated with an autologous transplant (Table 1). Although there is no accepted standard, there are 2 general approaches. The R-HyperCVAD alternating with high-dose methotrexate/cytarabine alternating with rituximab high-dose methotrexate/cytarabine regimen18 produces exceptionally high (87%) CR rates with durable responses.19 Although the single center experience is impressive with this regimen, it has not proved possible to replicate these results in a multi-institutional setting.20,21 The alternative approach has been to consolidate responses achieved with chemotherapy with an autologous transplant. Arguably the best multicenter results seen in this setting come from the Nordic group, which incorporated cytarabine as part of cytoreductive therapy before autograft.22 The progression-free survival (PFS) at 6 years was 70%,23 similar to results with HyperCVAD; however, it appears to be a less toxic regimen. What drugs need to be added to cytarabine in this context is not clear, but a recently reported large randomized trial clearly demonstrates the need for its incorporation as a component of the chemotherapy prior to an autologous transplant.24
For patients in whom an intensive approach to management is not feasible, there is no generally accepted front-line therapy (Table 2). There are a number of chemotherapeutic backbones that can be used in MCL, most commonly CHOP,25 fludarabine and cyclophosphamide (FC),26 and bendamustine.27 A large randomized study recently demonstrated a survival benefit for the use of R-CHOP over rituximab and FC (R-FC) in older patients with MCL.28 This study also demonstrated the clear benefit of rituximab as maintenance after R-CHOP, where it doubled remission duration in responding patients. The use of the R-CHOP/R-cyclophosphamide, vincristine, and prednisolone (CVP) regimens in comparison with R-bendamustine (R-B) has been assessed across a range of lymphomas including MCL in 2 randomized trials.29,30 For the MCL cohorts, the R-B combination demonstrated a superior PFS29 and response rate30 but no difference in OS. Neither trial involved rituximab maintenance; because the addition of this significantly improves the outcome following R-CHOP and there are currently no available data on maintenance after R-B, it is not clear which of these 2 regimens is superior.
For the more frail patient where it is not possible to use either of these approaches, a number of less intensive therapies are available, including rituximab31 alone as well as CVP,32 chlorambucil,33 cladribine,34 or thalidomide,35 usually in combination with rituximab.
At relapse, there is no standard of care. Generally, an alternative immuno-chemotherapeutic regimen from that used initially is given, but invariably the quality and durability of any remissions achieved are inferior to those seen after front-line therapy. For younger patients, allogeneic transplantation can be considered. There are conflicting data on both its efficacy and toxicity in this setting, reflecting the nature of the patients being treated, many of whom have failed a prior autograft and have relatively advanced disease.36,37 However, recent registry data have demonstrated a 2-year OS of 46% in patients receiving an allograft after a relapsed autograft, with those patients having an initial remission duration >1 year after autograft having the best outcomes.38
Improving on standard therapy
To improve on the existing outcomes with standard therapy requires either improving responses, producing more durable responses, or alternatively achieving the same results with less toxicity. For older patients, the toxicity associated with therapy and the attendant quality-of-life implications are very important in what is an incurable condition. This is well illustrated with the application of the highly active HyperCVAD regimen in patients >65 years of age. The toxicity associated with this regimen prevented its adoption for these patients,18 but modification of the original HyperCVAD regimen by omitting the methotrexate and cytarabine produced a CR rate of 64% in more elderly patients.39
A number of studies have evaluated the potential of alternative anti-CD20 monoclonal antibody approaches. As a single agent, rituximab induces responses of 35% in MCLs.31 With the advent of newer anti-CD20 monoclonal antibodies, these offer the potential to improve response rates. To date, the single agent data in MCLs are limited, but with response rates of 27% and 8.3% with obinutuzumab (GA 101)40 and ofatumumab,41 respectively, these drugs do not appear to offer a major advance; however, because most of these patients will have already received rituximab, a clear comparison is difficult. Some immuno-chemotherapy combination studies using these newer anti-CD 20 antibodies are ongoing.
90Y-ibritumomab-tiuxetan produces response rates of 31% in relapsed or refractory MCLs.42 Using ibritumomab-tiuxetan as consolidation therapy following an abbreviated course of R-CHOP therapy improves responses, producing a 55% CR/CR undetermined (CRu) rate and estimated 3-year survival of 80%.43 Although it uses less chemotherapy, these results are not better than R-CHOP followed by maintenance.28
The Nordic group incorporated ibritumomab-tiuxetan into their standard protocol,22 where it is given prior to the autograft in those patients who fail to achieve a CR. These data have recently been published44 and fail to demonstrate any benefit for the addition of this agent. In addition, the use of ibritumomab-tiuxetan as consolidation therapy following HyperCVAD leads to substantial and unacceptable toxicity.45 Taken together, it is not obvious that any of these agents offers a significant benefit over rituximab. An alternative approach is to monitor patients after autograft and treat preemptively with rituximab at molecular relapse.46 A more pragmatic approach based on the data in older patients after R-CHOP would be to use rituximab as maintenance therapy after front-line therapy.28 A randomized trial of rituximab use after autograft has been performed and the data are awaited (#NCT00921414).
Before considering newer agents, are there different ways of using the conventional drugs that may improve outcomes in older patients with MCLs? Cytarabine appears to be the key chemotherapeutic agent for younger patients. As a dose-response has not been established in MCLs, exploring lower-dose strategies in older patients would appear logical. The addition of cytarabine at a dose of 800 mg/m2 for 3 days with bendamustine and rituximab in elderly patients led to a CR of 95% in previously untreated patients.47 This produced significant hematological toxicity, and lower doses are now being used. In another study, cytarabine (1 g/m2 for 4 doses per cycle) with rituximab alternating with R-CHOP followed by 2 cycles of cytarabine given with fludarabine was given to 60 elderly patients with a CR/CRu of 87%.48 This agent would appear to have a role in older patients, and further dose exploration perhaps with doses used in the elderly acute leukemia setting may be interesting.
Newer agents
There are currently 4 drugs licensed for use in MCLs: bortezomib (Velcade), temsirolimus (Torisel), lenalidomide (Revlimid), and most recently ibrutinib (Imbruvica). The relative activity of these agents in relapsed and refractory MCLs as reported in the trials on which registration was based is shown in Table 3. Although one can argue that the treated populations are not completely comparable, this does give a reasonable impression of the relative activity of these agents. At the time of writing, temsirolimus is the only agent that is licensed within Europe, because randomized comparative evidence has been a requirement for registration in this region. It is also the only agent of the 4 that does not have a license in the United States. As single agents, the ORRs for these drugs are 33% (8% CR) with bortezomib,49 22% (2% CR) with temsirolimus,50 28% (8% CR) with lenalidomide,51 and 68% with ibrutinib (21% CR).16 With the possible exception of ibrutinib, it seems unlikely that these agents will be used as single agents for the treatment of MCLs outside of maintenance strategies.
Temsirolimus appears to be the least active of these drugs when used as a single agent. There are some ongoing trials with this agent in combination with chemotherapy, but the only published data are in combination with rituximab,52 where the ORR was 60% (CR 19%) and the duration of response (DOR) was 11 months.
Bortezomib has been incorporated into many regimens (Table 4) and is the subject of an ongoing cooperative study in the United States (#NCT01415752). As part of front-line therapy, the incorporation of bortezomib within R-HyperCVAD53 (90% CR), R-CHOP54 (72% CR/CRu), and rituximab, bortezomib, modified HyperCVAD (VcR-CVAD)55 (77% CR/CRu) appears to be a significant advance over the original regimens. The use of subcutaneous bortezomib reduces the incidence of neuropathy, which can be a significant problem,56 and its use should become standard. A very large international randomized trial studying the addition of bortezomib to R-CHOP (#NCT00722137) has completed recruitment and should provide definitive evidence for the value of this agent within front-line therapy. The addition of bortezomib to cytarabine is synergistic in vitro,57 and the combination shows activity in relapsed patients.58 The addition of bortezomib either within the original HyperCVAD regimen53 or within a less intensive variation59 looks highly promising without significantly increasing toxicity. An extension of the initial Chang study59 included 75 patients that we offered a choice of consolidation therapy between an autograft and rituximab maintenance. Although not a randomized trial, there was no obvious difference between these 2 approaches, questioning the value of an autograft in this setting.
Lenalidomide is an immuno-modulatory drug that has activity as a single agent when used in MCLs. When combined with rituximab, response rates appear to be increased, with an ORR of 57% (36% CR) observed in 52 patients.60 In the phase 1 component of this study, the established maximal tolerated dose of lenalidomide was 20 mg. Another study used low-dose maintenance lenalidomide at 15 mg in patients who had responded at the conventional dose of 25 mg daily.61 Some durable responses were observed in this study: a DOR of 22 months and fewer side effects seen when the dose was lowered for the maintenance phase. Studies using lenalidomide as maintenance with or without rituximab are ongoing, as well as some combination studies involving bendamustine and rituximab and some of the newer agents.
It is beyond the scope of this article to discuss in detail the plethora of agents that may have a future role in the management of this disease; this topic has been reviewed elsewhere.14,15 However, targeting the BCR and PI3K-AKT-mTOR signaling pathways would appear to be the most promising areas therapeutically at the moment.
Targeting mTOR does not appear very efficacious as already observed with temsirolimus and more recently with everolimus, where an ORR of only 8.6% (CR 0%) was observed in 58 patients with relapsed/ refractory disease.62 Conversely, PI3K and Bruton’s tyrosine kinase (BTK) inhibition appear more promising. Idelalisib is a first in class, highly selective, orally administered inhibitor targeted against PI3K-δ. PI3K-δ is critical for multiple signaling pathways that are hyperactive in B-cell malignancies including MCLs. The phase 1 study of idelalisib in MCLs has recently been published63 and included 40 heavily pretreated patients. Although many patients responded, the documented ORR was 40% (CR 5%) but with a median DOR of only 2.7 months and median PFS of 3.7 months. Although 25% patients discontinued therapy due to adverse effects, the toxicity profile appears favorable with no clear myelosuppression and no identified maximal tolerated dose. This agent is well suited to combination approaches, and a number of studies are ongoing, the most advanced being with everolimus, bortezomib, and bendamustine/rituximab.
The drug that appears to have the most activity when used as a single agent in MCLs is ibrutinib (Imbruvica). This is a first in class orally bioavailable BTK inhibitor. BTK is a major component and mediator of BCR signaling.64 The initial phase 1 trial with this agent involved patients with B-cell non-Hodgkin’s lymphoma65 and included 9 patients with MCLs, 7 of whom demonstrated a response. The subsequent phase 2 trial of 111 heavily pretreated patients with MCLs showed an ORR of 68% (CR 21%) and a median PFS of 13.9 months.16 The responses observed in this trial demonstrated different kinetics to those traditionally seen with chemotherapy, with some patients taking up to 9 months to enter remission. The complete remission rate doubled in the study when measured at 12 months compared with 4 months (20.7% vs 9%), although the ORR was similar (66.7% vs 62.2%). This drug has a modest side effect profile, with the most common treatment-related adverse events being mild or moderate diarrhea, fatigue, and nausea. Grade ≥3 hematologic events occurred in <17% of patients. In common with the PI3K inhibitors, some patients experience a redistribution phenomenon following treatment, with a lymphocytosis occurring as tumor cells exit other tissue compartments. This is relatively short lived but can be dramatic. The very modest hematologic toxicity permits the incorporation of ibrutinib into standard immuno-chemotherapy regimens. Many trials in evolution are incorporating ibrutinib within many of the currently adopted active regimens. Ultimately, the question will be whether ibrutinib by itself or together with an antibody can be used in place of chemotherapy as front-line therapy. Based on the clear evidence of activity observed not only in MCLs but also in other lympho-proliferative disorders, there are currently in excess of 10 BTK inhibitors at varying stages of clinical development.66 With the recent in vitro recognition of potential mechanisms of insensitivity to ibrutinib via activation of an alternative NF-κB pathway,13 this points to the potential for molecular profiling of tumors prior to therapy to help direct targeted treatments, including combinations.
Stratified approaches
With the therapeutic options that are available, is it possible to tailor therapy based on known prognostic factors or adjust therapy based on response criteria? There are a number of known prognostic factors in MCLs67 ; however, only the mantle cell international prognostic index68 and Ki679 are generally available and potentially useful. Although these are robust indices and define risk over a range of clinical trials and population-based studies, for the majority of patients they cannot be used to tailor therapy. The only place where this may not apply is in the younger patient where a high-risk mantle cell international prognostic index, high Ki67, or blastoid variant disease may justify a more intensive approach to front-line therapy. However, this is not an evidence-based statement but merely reflects a comment on the relatively poor outcomes for these patients.
There is a group of patients whose disease behaves with a more indolent clinical phenotype, and for this cohort, an initial watch-and-wait strategy may be appropriate.69 Although these patients most commonly present with a leukemic component and there are some potential factors to help distinguish them,3 they are not well characterized. As yet, there is no evidence to support a different therapeutic approach for these patients at the time treatment is required; however, when there is a reliable way of distinguishing them, studies with less intensive therapies would seem logical.
Minimal residual disease (MRD) assessment can predict for outcome after therapy, when applied in a trial setting,70 involving limited laboratories, and with rigorous application of quality control. Outside of a trial setting, there does not seem to be a routine place for its use at the moment. The application of MRD in a maintenance setting has already been discussed. However, it may become a requirement to guide starting or stopping therapy based purely on the potential cost of the newer evolving maintenance strategies. Trials are beginning to explore the use of MRD as a guide to stratifying therapy. With the potential for highly active combinations involving BTK inhibitors, there may be a role to use MRD assessment as a basis to make a decision as to whether to proceed to high-dose therapy or whether to add newer drugs into ongoing chemotherapy.
Positron emission tomography (PET) scanning is widely applied in patients with lymphoma, and there is a growing body of evidence to direct therapy using a response-adapted approach. MCL is a PET-positive tumor; however, PET scanning does not reliably detect bone marrow or bowel disease and therefore does not add to conventional staging.71,72 In 3 large studies involving patients treated with different regimens, a positive PET scan did not predict for an inferior OS when used either as an interim assessment or at the end of therapy.71,73,74 One of these studies did show a significant difference in PFS following a positive PET at the completion of therapy,74 and 1 other smaller study found a positive post-therapy scan was more likely to predict for relapse.72 When used for surveillance, a high false-positive rate (35%) has been reported,71 and in 2 studies where surveillance was assessed, it was concluded that PET scanning did not meaningfully contribute.71,75 It is not clear what role PET scanning currently has in this disease, but it is clear that a change of therapy should not be made based on an interim assessment, and it has no role in surveillance. The significance of a positive PET after therapy is sufficiently unclear, suggesting that response criteria based on PET scanning should be applied with great caution in this disease.
Conclusion
For many years, there has been no significant advance in the management of patients with MCLs. In the younger patient, cytarabine-based therapy followed by an autologous transplant has become established as the standard of care, and in more elderly patients, CHOP and bendamustine have been widely used. The use of rituximab has made an impact but perhaps not as much as in more common lymphoma subtypes. We still do not have prognostic tools that routinely guide treatment decisions or robust diagnostic criteria for the more indolent patients. However, it is clear that we are entering a period of significant therapeutic advances. As the evidence accumulates, newer drugs will become incorporated into some of the standard therapeutic regimens. Bortezomib is likely to be the first of these. Maintenance strategies will become established; in addition to rituximab, other drugs such as lenalidomide may have a significant role. However, the application of BTK inhibitors would appear to be a step change in the therapy for this disease. It seems likely that over the next couple of years the treatment paradigm will fundamentally change as the evidence base for ibrutinib grows, and this offers the very real prospect that BTK inhibitors may obviate the need for transplantation in younger patients and even the need for chemotherapy in older patients. However, the challenge for cooperative trial groups around the world is how do we explore the very many potential new therapeutic options that the new agents present us with but in a limited population of patients.
Acknowledgments
E.C. is supported by the Spanish Ministry of Economy and Competitivity (SAF10/21165), Institució Catalana de Recerca i Estudis Avançats-Academia (ICREA-Academia), and Red Temática de Investigación Cooperativa en Cáncer (RD12/0036). S.R. is supported by Leukaemia and Lymphoma Research and Cancer Research UK.
Authorship
Contribution: E.C. wrote the section on pathogenesis of MCL; and S.R. wrote the rest of the article including generating all of the figures.
Conflict-of-interest disclosure: S.R. has acted as a consultant for Pharmacyclics, Roche, J&J, and Celgene and received research funding from Celgene and GSK. E.C. declares no competing financial interests.
Correspondence: Simon Rule, Department of Clinical Haematology, Plymouth University Peninsula School of Medicine and Dentistry, Plymouth PL6 8DH, United Kingdom; e-mail: simon.rule@nhs.net.