Abstract
We analyzed cost-effectiveness studies related to hematologic malignancies from the Tufts Medical Center Cost-Effectiveness Analysis Registry (www.cearegistry.org), focusing on studies of innovative therapies. Studies that met inclusion criteria were categorized by 4 cancer types (chronic myeloid leukemia, chronic lymphocytic leukemia, non-Hodgkin lymphoma, and multiple myeloma) and 9 treatment agents (interferon-α, alemtuzumab, bendamustine, bortezomib, dasatinib, imatinib, lenalidomide, rituximab alone or in combination, and thalidomide). We examined study characteristics and stratified cost-effectiveness ratios by type of cancer, treatment, funder, and year of study publication. Twenty-nine studies published in the years 1996-2012 (including 44 cost-effectiveness ratios) met inclusion criteria, 22 (76%) of which were industry funded. Most ratios fell below $50 000 per quality-adjusted life-years (QALY) (73%) and $100 000/QALY (86%). Industry-funded studies (n = 22) reported a lower median ratio ($26 000/QALY) than others (n = 7; $33 000/QALY), although the difference was not statistically significant. Published data suggest that innovative treatments for hematologic malignancies may provide reasonable value for money.
Introduction
During the past 15 years, treatment of hematologic malignancies changed radically. In 1997, the US Food and Drug Administration approved rituximab, now widely used to treat hematologic malignancies, including non-Hodgkin lymphoma (NHL). Later, tyrosine kinase inhibitors (TKIs) were introduced to treat chronic myeloid leukemia (CML). TKIs exceeded survival benefit expectations1 ; however, they also have a notably high cost.
The first TKI, imatinib, was introduced in 2001 at roughly $30 000/y of treatment. Others, introduced more recently, cost roughly $100 000/y or more.2 These prices have prompted significant outcry, with some questioning whether these medications provide good value for money.2 The use of bortezomib (a proteasome inhibitor) and the novel antiangiogenesis agent lenalidomide have improved multiple myeloma (MM) outcomes. These and other innovative treatments have increased patients’ life expectancy.1,3
Cost-effectiveness analysis (CEA) is a technique to assess the benefit of interventions relative to their costs. Cost-utility analysis (CUA) is a type of CEA that measures health benefits in quality-adjusted life-years (QALYs). This generic measure facilitates comparison of health care interventions addressing varied conditions. CUA has been used extensively in oncology.4
We identified innovative treatments for hematologic malignancies and performed a systematic review of peer-reviewed CUAs. It is important to understand the current literature regarding these treatments, which have undergone dramatic changes in cost and effectiveness. We synthesized analyses of care for hematologic malignancies; examined the number, quality, and related characteristics of analyses; and summarized cost-utility ratios by treatment and disease types. To our knowledge, this review is the first of its kind.
Methods
We analyzed data from the Tufts Medical Center CEA Registry (www.cearegistry.org), a database of over 9800 cost-effectiveness ratios published in the peer-reviewed medical and economic literature through 2012. The Registry’s development and inclusion criteria are described elsewhere.5 Briefly, English-language publications identified by a MEDLINE search that contain an original cost per QALY estimate were retrieved. Two trained researchers evaluated articles for inclusion and extracted article information. Disagreement was resolved by consensus.
Our review included studies that addressed treatment of hematologic malignancies. We excluded review, editorial, or methodologic articles; CEAs that did not measure health effects in QALYs; and non-English-language articles. We included therapeutic agents that the US Food and Drug Administration approved since 1997 and excluded hematopoietic stem cell transplant, symptom management, and supportive care. The studies were manually checked for duplication. No duplicates were found.
We collected data regarding study origin, methods, and reporting of results. For each CUA, descriptive characteristics collected included publication year, country of origin, intervention type, publication journal, funding source, and methodologic and analytic characteristics including study perspective, discounting of future costs and life-years, whether economic data were collected alongside a clinical trial, and type of sensitivity analysis performed (ie, univariate, multivariable, or probabilistic). Each study was assigned a subjective quality score on a Likert scale from 1 (low) to 7 (high), based on rigor of methodology, presentation, and value to decision makers. We conducted a subgroup analysis of incremental cost-effectiveness ratio (ICER) distributions that included only quality scores that were high (≥5) and compared this analysis to the distribution in all studies.
To facilitate comparison of standardized outcomes, non–United States currencies were converted into United States dollars using the appropriate foreign exchange factor for the relevant year, and ratios were inflated to 2012 dollars using the general Consumer Price Index.
We grouped studies into subcategories by 4 cancer types (chronic lymphocytic leukemia, CML, MM, and NHL) and 9 treatment agents (interferon-α, alemtuzumab, bendamustine, bortezomib, dasatinib, imatinib, lenalidomide, rituximab alone or in combination, and thalidomide). Some studies contributed multiple ICERs, because they compared several interventions or included scenarios for multiple settings. For analysis, we assigned each ratio a statistical weight of 1 divided by the number of ratios contributed by that study.
Results and discussion
Table 1 describes the characteristics of the 29 studies (published in 1996-2012; supplemental Appendix 1, available on the Blood Web site) analyzed in this review. The journals that published the highest number of these studies were Leukemia & Lymphoma (5 studies), Pharmacoeconomics (4), Value in Health (4), and European Journal of Haematology (3). Nine studies were conducted from a United States perspective, followed by the United Kingdom (6), Norway (3), Sweden (3), and France (2). Most studies (18) were conducted from a health care payer perspective. Seven were conducted from a societal perspective (as recommended by the US Panel on Cost-Effectiveness and Health in Medicine6,7 ). The pharmaceutical industry funded 22 studies (76%). Six studies collected economic data alongside a clinical trial. The mean quality rating was 4.85 (±0.61) (the mean rating overall in the Registry was 4.45 [±1.03]). The mean quality rating was analyzed using a 2-tailed Student t test and did not differ significantly between industry- and non-industry-funded studies.
The studies reported 44 cost-effectiveness ratios (some of the 29 studies reported multiple ratios). Most ratios addressed interventions for NHL (41%) or CML (30%). Most ratios pertained to treatments for rituximab (43%), interferon-α (18%), or imatinib (16%). The most common intervention-disease combination was rituximab alone or in combination for NHL (36%).
Across cancers, the median reported ratio was highest for CML ($55 000/QALY) and lowest for NHL ($21 500/QALY). Median reported ratios fluctuated across time periods: $35 000/QALY (1996-2002), $52 000/QALY (2003-2006), and $22 000/QALY (2007-2012). The median ratio reported by industry-funded studies ($26 000/QALY) was lower (more favorable) than the median reported by non-industry-funded studies ($33 000/QALY). These differences were analyzed using a χ2 test, and results for trends across time periods were confirmed with a logistics regression; the differences were not statistically significant.
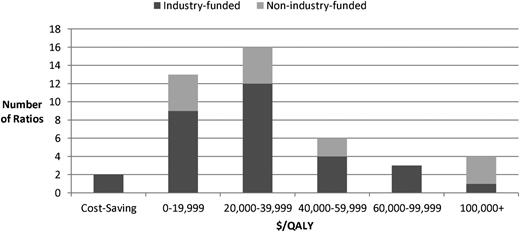
Most ratios fell below (were more favorable than) the $50 000/QALY (73%) or $100 000/QALY (86%) threshold (Figure 1). These thresholds are commonly used in the United States as benchmarks for cost effectiveness. Although their origins are unclear, evidence suggests that thresholds at these levels or higher may be appropriate depending on the context.8 (A higher threshold would expand the range of analyses considered cost effective, which would mean that our analysis is conservative and that more of the CEAs included should be considered cost effective.) Four ratios, 1 of them industry funded, exceeded $100 000/QALY, including 2 pertaining to treatment of MM with bortezomib,9,10 1 pertaining to treatment of CML with interferon-α,11 and 1 pertaining to treatment of CML with imatinib. In 2 other cases, both industry funded (alemtuzumab to treat chronic lymphocytic leukemia and bortezomib to treat MM), the treatment improved health and reduced costs.
Ratios by ICER ($/QALY) and funding source. Note: ratios were inflated to 2012 United States dollars using the general Consumer Price Index.
Ratios by ICER ($/QALY) and funding source. Note: ratios were inflated to 2012 United States dollars using the general Consumer Price Index.
The distribution of ICERs by value in the high-quality subsample (quality scores ≥5) was similar to the distribution in all studies, yielding findings that were not significantly different than those in the overall analysis. For example, when comparing ICERs from industry- and non-industry-funded studies in the high-quality subsample, there was not a statistically significant difference in the number of ICERs that had values ≥$100 000/QALY.
Our review suggests that many new treatments for hematologic malignancies may confer reasonable value for money. Despite the high costs of new drugs, the cost-effectiveness ratio distributions are comparable to those for cancers overall and to other health care fields.4,12 For example, a 2010 study found that the majority of published cost-effectiveness ratios reported for cancer interventions were <$50 000/QALY.4 As in similar reviews, our results may reflect publication bias.12 In particular, they may reflect selective conduct of studies and underreporting of unfavorable findings, particularly by industry-funded studies, which made up the majority of the reviewed studies.12
Treatments for NHL may appear more cost effective than treatment for CML, because innovative agents, such as rituximab for NHL, were introduced in earlier years than TKIs for CML, which may have affected the manufacturers’ pricing strategies. In addition, unlike rituximab, the TKI market has experienced a flurry of agents approved for the same target. Churning in this market, based on loss of efficacy, concern about gene mutation, differences in dosing administration (eg, daily vs twice daily), and side effect profiles, may result in increasing costs for CML treatment as compared to NHL.
We believe that this kind of global overview of the literature will be useful to provide a broad perspective for clinicians on how their individual focus relates to associated interventions and malignancies. In the context of recent discussions about the cost effectiveness of various interventions in the popular media, it may be particularly useful for clinicians to understand the literature in the field as a whole. We appreciate that individual clinicians may concentrate on specific malignancies and therapies, and we have therefore included data that we hope will be useful in these circumstances regarding the proportion of studies and median ratios for specific diseases and interventions.
Faced with rising costs, many payers use economic analyses to inform coverage decisions; frequently, they limit access to expensive new drugs.13 For example, after reviewing the cost-effectiveness evidence, the United Kingdom’s National Institute for Health and Clinical Excellence recently approved nilotinib, a second-generation TKI, but only after the manufacturer discounted it.14
Our study has several limitations. First, our review includes only CEAs in the Tufts Medical Center CEA Registry and, therefore, only those that quantified health benefits in QALYs. Other evaluations may have used other measures such as life-years. Second, our review was limited to English-language peer-reviewed publications indexed in MEDLINE. We did not include health technology assessment reports. Third, we did not evaluate the analyses’ clinical or modeling assumptions or assess the quality of data collected in studies conducted alongside clinical trials. Alternate approaches to quality assessment have been provided in the literature, such as the Consolidated Health Economic Evaluation Reporting Standards.15 Finally, the ratios depend on cost and benefit assumptions that may have changed since publication. For example, Novartis increased the price of imatinib from ∼$30 000 per year of treatment in 2001 to roughly 3 times that amount as of 2012.2 A CUA would therefore evaluate imatinib as more cost effective in 2001 than it would in 2012 at its higher price.
In summary, many new treatments for hematologic malignancies appear to be cost effective based on the published literature. Industry-funded studies reported a lower (more favorable) median ratio than non-industry-funded studies. However, both groups reported medians <$50 000/QALY. Although prices are high, the treatments confer substantial health benefits as measured by QALYs. However, decision makers consider factors other than cost effectiveness (eg, health impact, values, preferences, overall budget impact, affordability for patients) and determine how best to weight these factors according to the situation at hand.16,17 Decision makers can use CEA as a tool to help determine appropriate coverage for these and other drugs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by internal sources at the Center for the Evaluation for Value and Risk in Health at the Institute for Clinical Research and Health Policy Studies at Tufts Medical Center in Boston. Research reported in this publication was also supported by the National Cancer Institute of the National Institutes of Health under award number T32 CA009429 (S.K.P., G.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: A.N.W. and C.J.S. designed the research and collected and analyzed the data; G.S. collected the data and provided clinical input; S.K.P. designed the research and provided clinical input; P.-J.L. designed the research; J.T.C. and P.J.N. directed and designed the research; and all authors contributed to the analysis and interpretation of the results and wrote the paper.
Conflict-of-interest disclosure: The Center for the Evaluation of Value and Risk in Health receives funding from government, private foundation, and industry sources. The authors have no further conflicts of interest to disclose.
Correspondence: Peter J. Neumann, Center for the Evaluation of Value and Risk in Health, Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, 800 Washington St, Box 63, Boston, MA 20111; e-mail: pneumann@tuftsmedicalcenter.org.