To the editor:
Polycythemia vera (PV) and essential thrombocythemia (ET) are characterized by JAK2V617F mutation in 95% and 60% of the patients, respectively.1 Ruxolitinib is a JAK1/JAK2 inhibitor approved for myelofibrosis (MF) and more recently for hydroxyurea resistant/intolerant PV patients because of its superiority to standard therapy (and placebo in MF) in improving splenomegaly, ameliorating symptoms, and reducing phlebotomies (in PV).2 A modest reduction of the JAK2V617F allele burden (8% from baseline at 72 weeks) was observed in MF patients in the COMFORT-II study.3 A progressive decrease of the JAK2V617F allele burden by a mean of 22% at 36 months was reported in 34 PV patients enrolled in a phase 2 trial (INCB18424-256, ClinicalTrials.gov #NCT00726232) that also included 22 ET patients, with 23.5% of the patients achieving a ≥50% reduction; however, no complete molecular remission (CMR) was attained at that time.4 Comparably, in the phase 3 RESPONSE study in PV, the mean decrease of JAK2V617F allele burden at week 32 (n = 92) and at week 112 (n = 22) was 12.2% and 34.7%, respectively.2
Twenty-two JAK2V617F-mutated patients, 11 PV and 11 ET, were enrolled in our center in the INCB18424-256 study, and 19 have been followed for >5 years. We measured the JAK2V617F allele burden by 2 reverse-transcriptase quantitative polymerase chain reaction (RT-qPCR) assays (sensitivities of 0.8% and 0.08%),5 and deep amplicon resequencing (Ion Torrent platform).6 Approval was obtained from the Azienda Ospedaliero-Universitaria Careggi institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
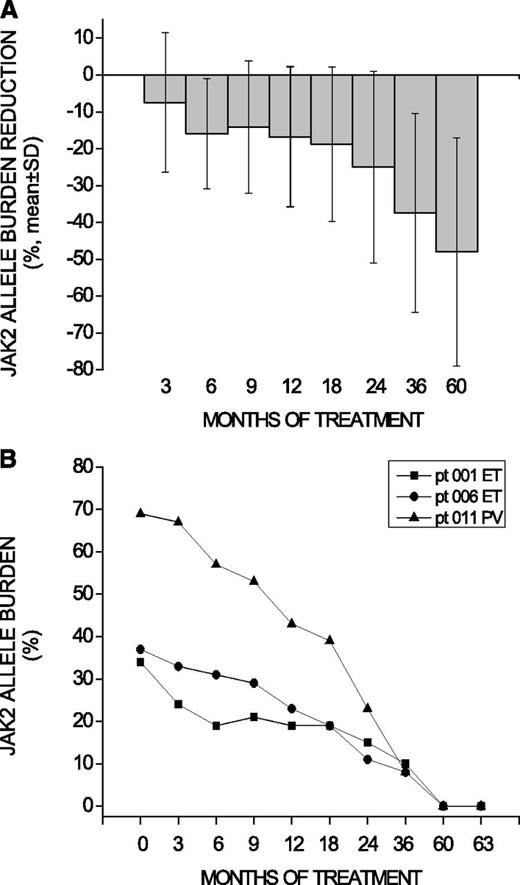
Overall, JAK2V617F allele burden decreased by a mean of 19% and 28% from baseline at 36 and 60 months, respectively, consistent with previous reports.2,4 Among 13 patients showing a sustained reduction of the allele burden >25% at 60 months, the mean decrease was 48% (Figure 1A). Notably, 3 patients (1 PV, 2 ET) achieved a ≥50% allele burden reduction after 2 years, and progressed to a CMR at 5 years (Figure 1B). Their mean allele burden was 46.6% at baseline and 28.3%, 16.3%, 8.7%, and 0% at 1, 2, 3, and 5 years, respectively (Figure 1B). A JAK2V617F CMR status was confirmed by both RT-qPCR assays and deep resequencing 3 months after the first observation. The 3 patients had normal karyotype both at baseline and at 5 years, and the 2 ET patients were MPL and CALR wild-type. Additionally, a TET2 Y867H mutation with an allele burden of 48.9% at baseline, remaining unchanged at 5 years (52%), was found in the PV patients.
JAK2V617F allele burden decrease in patients achieving >25% reduction at 60 months and details of patients attaining complete molecular remission. (A) The percentage decline over time (mean ± SD) of the JAK2V617F allele burden in the 13 patients who presented a >25% allele burden reduction at 60 months. JAK2V617F allele burden decreased by a mean of 7%, 11%, 19%, and 28% at 1, 2, 3, and 5 years, respectively. (B) The absolute level of JAK2V617F allele burden in the 3 patients (pt) who finally achieved a CMR at 5 years (confirmed 3 months later) is presented. Measurement of the JAK2V617F allele burden was performed in peripheral blood granulocytes by RT-qPCR. The attainment of CMR was further confirmed by both a high-sensitivity RT-qPCR assay (detection limit, 0.08%) and deep resequencing at 5 years and at +3-month time points. SD, standard deviation.
JAK2V617F allele burden decrease in patients achieving >25% reduction at 60 months and details of patients attaining complete molecular remission. (A) The percentage decline over time (mean ± SD) of the JAK2V617F allele burden in the 13 patients who presented a >25% allele burden reduction at 60 months. JAK2V617F allele burden decreased by a mean of 7%, 11%, 19%, and 28% at 1, 2, 3, and 5 years, respectively. (B) The absolute level of JAK2V617F allele burden in the 3 patients (pt) who finally achieved a CMR at 5 years (confirmed 3 months later) is presented. Measurement of the JAK2V617F allele burden was performed in peripheral blood granulocytes by RT-qPCR. The attainment of CMR was further confirmed by both a high-sensitivity RT-qPCR assay (detection limit, 0.08%) and deep resequencing at 5 years and at +3-month time points. SD, standard deviation.
At the time of CMR, the PV patient was in complete hematologic remission.4 However, bone marrow evaluation showed normalization of myeloid and megakaryocyte lineage but persistence of erythroid hyperplasia. Conversely, at the time of CMR, the 2 ET patients were in partial hematologic remission due to their platelet counts of 422 × 109/L and 812 × 109/L; the bone marrow biopsy at 5 years showed slight megakaryocyte hyperplasia without morphologic abnormalities and no fibrosis. To exclude selective persistence of the JAK2V617F mutation in the megakaryocyte lineage, we evaluated the mutation in platelet-rich plasma RNA but found no evidence of it.7
Until now, JAK2V617F CMR has been reported in 14% to 24.1% of PV patients and 6% to 17% of ET patients receiving interferon8 and in 9% of MF patients treated with imetelstat.9 Reported effects of hydroxyurea were variable, with some series reporting CMR in 12% to 26% of ET patients and in 8% to 17% of PV patients, whereas other studies showed only modest allele burden decrease in few patients.10,11
Our data confirmed the overall modest JAK2V617F allele burden reduction seen in previous studies in patients receiving ruxolitinib but indicated that some (16% in this series) may attain a JAK2V617F CMR with prolonged treatment. The persistence of other mutations, such as TET2, may suggest biclonal disease or a single ancestral TET2-mutated founder clone later acquiring JAK2V617F; we could not distinguish between these 2 possibilities, lacking viable cells for clonal analysis.
The attainment of JAK2V617F CMR occurring notwithstanding the persistence of some PV- and ET-associated features might reflect either additional clone(s) with unknown mutations insensitive to JAK2 inhibition or different kinetics of normalization of histologic and hematologic parameters. Larger studies are required to establish the frequency of CMR with JAK2 inhibitors and its relevance for the management of these chronic diseases.
Authorship
Acknowledgments: This study was supported by a special grant (#1005) from the Associazione Italiana per la Ricerca sul Cancro “AIRC 5 per Mille” to the AIRC-Gruppo Italiano Malattie Mieloproliferative (www.progettoagimm.it); grant #RBAP11CZLK from the Fondo per gli Investimenti della Ricerca di Base (FIRB2010); and grant #GR-2001-02352109 from the Ministero della Salute.
Contribution: L.P. collected the data, performed the data analysis, and wrote the manuscript; A. Pancrazzi and T.F. performed the laboratory and data analysis; A. Pacilli, C.R., and G.R. performed the laboratory analysis; P.G., R.F., C.P., and S.V. collected the data; and A.M.V. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: A.M.V. received honoraria from Novartis for serving on the advisory board and for lectures, and received research funding from Novartis to the University of Florence. S.V. received research support for conducting a clinical study by Incyte. The remaining authors declare no competing financial interests.
Correspondence: Lisa Pieri, Laboratorio Congiunto MMPC, Department of Experimental and Clinical Medicine, University of Florence, Largo Brambilla 3, 50134 Florence, Italy; e-mail: lisa.pieri@unifi.it.