Abstract
Blood is a tissue with high cellular turnover, and its production is a tightly orchestrated process that requires constant replenishment. All mature blood cells are generated from hematopoietic stem cells (HSCs), which are the self-renewing units that sustain lifelong hematopoiesis. HSC behavior, such as self-renewal and quiescence, is regulated by a wide array of factors, including external signaling cues present in the bone marrow. The transforming growth factor-β (TGF-β) family of cytokines constitutes a multifunctional signaling circuitry, which regulates pivotal functions related to cell fate and behavior in virtually all tissues of the body. In the hematopoietic system, TGF-β signaling controls a wide spectrum of biological processes, from homeostasis of the immune system to quiescence and self-renewal of HSCs. Here, we review key features and emerging concepts pertaining to TGF-β and downstream signaling pathways in normal HSC biology, featuring aspects of aging, hematologic disease, and how this circuitry may be exploited for clinical purposes in the future.
Introduction
Blood cell production takes place in the bone marrow (BM) of adult individuals, where it originates from a rare pool of tissue-specific stem cells known as hematopoietic stem cells (HSCs). HSCs function to sustain and regenerate the entire blood system in an unbroken fashion throughout life, as they have the dual capacity to self-renew and differentiate to all blood cell lineages.1,2 Self-renewal pertains to the ability of a stem cell to duplicate itself without losing developmental potential. This capacity is crucial for maintenance of the stem cell pool and may occur in a symmetric or asymmetric fashion.3 Furthermore, quiescence, or withdrawal from the cell cycle, is a feature of HSCs that provides the blood system with a dormant HSC reservoir that retains the ability to self-renew and replenish all blood lineages. Hematopoiesis is hierarchically organized, with the most immature pool of HSCs at the top, giving rise to various progenitor cells that progressively lose self-renewal capacity as they form differentiating progeny that proliferate extensively, finally generating functional blood cells at the bottom of the hierarchy (Figure 1). To rapidly tailor blood production to acute changes, such as bleeding and infection, HSC behavior is tightly regulated yet highly flexible.4 Signals that promote quiescence, and cues that stimulate proliferation and differentiation, provide a fine-balanced system that safeguards against depletion and overproduction of HSCs. Disruption of these regulatory mechanisms can result in blood disease, such as BM failure or leukemia. Transforming growth factor-β (TGF-β) is the founding member of a large family of secreted polypeptide growth factors, consisting of over 30 members in humans, including activins, bone morphogenetic proteins (BMPs), and others.5 The TGF-β family constitutes a multifunctional set of cytokines that regulate a bewildering array of cellular processes during development and beyond. In the adult organism, TGF-β members regulate tissue homeostasis and regeneration. With respect to hematopoiesis, TGF-β plays an important role in regulating HSC behavior, particularly quiescence and self-renewal. Although many members of this family function to regulate hematopoiesis, we focus this review mainly on TGF-β.
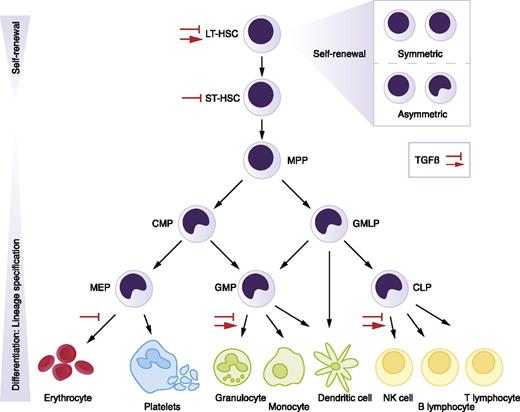
The hematopoietic hierarchy. Hematopoiesis is organized in a hierarchical manner, with rare HSCs at the top that give rise to various types of progenitor cells, which proliferate extensively, finally generating mature blood cells at the bottom of the hierarchy. Red arrows indicate stimulation of proliferation by TGF-β, whereas inhibition signs point to TGF-β’s growth inhibitory effect, in specific cell types or lineages. CLP, common lymphoid progenitor; CMP, common myeloid progenitor; GMLP, granulocyte-macrophage-lymphocyte progenitor; GMP, granulocyte-macrophage progenitor; LT-HSC, long-term HSC; MEP, megakaryocyte-erythrocyte progenitor; MPP, multipotent progenitor; NK, natural killer; ST-HSC, short-term HSC.
The hematopoietic hierarchy. Hematopoiesis is organized in a hierarchical manner, with rare HSCs at the top that give rise to various types of progenitor cells, which proliferate extensively, finally generating mature blood cells at the bottom of the hierarchy. Red arrows indicate stimulation of proliferation by TGF-β, whereas inhibition signs point to TGF-β’s growth inhibitory effect, in specific cell types or lineages. CLP, common lymphoid progenitor; CMP, common myeloid progenitor; GMLP, granulocyte-macrophage-lymphocyte progenitor; GMP, granulocyte-macrophage progenitor; LT-HSC, long-term HSC; MEP, megakaryocyte-erythrocyte progenitor; MPP, multipotent progenitor; NK, natural killer; ST-HSC, short-term HSC.
The basic elements of TGF-β signaling
TGF-β ligands signal through cell surface serine/threonine kinase receptors, known as type I and type II receptors.6 In vertebrates 7 different type I receptors (activin receptor-like kinase [ALK1-7]) and 5 distinct type II receptors have been identified, serving the entire family of ligands.6 TGF-β signals mainly via the type I receptor, ALK5, and the type II receptor, TβRII, both of which are required for signaling activation. Following ligand binding and receptor phosphorylation, the SMAD signaling circuitry becomes activated (Figure 2).6 The SMAD proteins are a family of transcription factors consisting of 8 members, SMAD1-8, which are further subdivided into 3 classes based on structural and functional properties.7 Receptor-regulated SMADs (R-SMADs), SMAD1, 2, 3, 5, and 8, are the only SMADs directly phosphorylated and activated by the kinase domain of type I receptors. Upon phosphorylation, R-SMADs form a complex with the common SMAD, SMAD4, resulting in nuclear accumulation of activated complexes. In the nucleus, R-SMAD–SMAD4 complexes cooperate with transcriptional coregulators that further define target gene recognition and transcriptional regulation.7 The inhibitory SMADs, SMAD6 and SMAD7, constitute the third class, which function to inhibit TGF-β signaling. TGF-β/activin/nodal and BMP/growth differentiation factor use different subsets of R-SMADs. R-SMAD2/3 specifically relay signals from TGF-β and activin receptors, whereas R-SMAD1/5/8 primarily operate downstream of BMP receptors.6
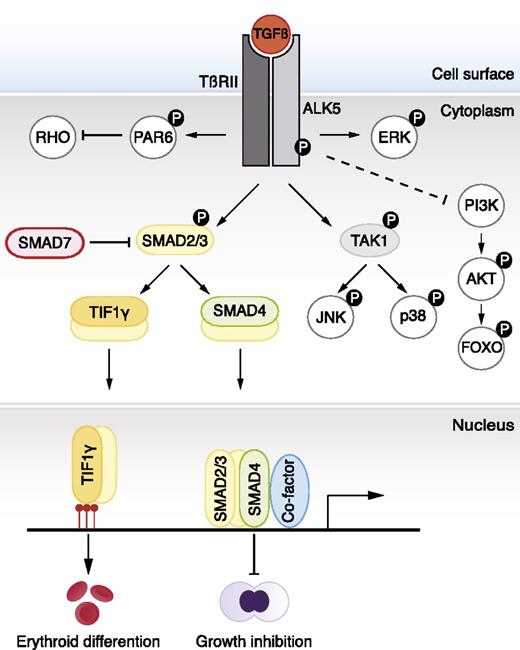
TGF-β signaling pathways. TGF-β ligands bind type I and type II receptors at the cell surface. Subsequently, the type I receptor (ALK5) becomes phosphorylated by the type II receptor. This leads to phosphorylation of SMAD2 and SMAD3, which form a complex with SMAD4. Activated complexes accumulate in the nucleus where they cooperate with DNA-binding cofactors to regulate target gene transcription. SMAD2 and SMAD3 also bind to TIF1γ. In embryonic stem cells, SMAD2/3-TIF1γ recognizes specific chromatin marks, promoting access of SMAD2/3-SMAD4 to otherwise repressed targets. TIF1-γ–SMAD2/3 promotes erythroid differentiation whereas SMAD4-SMAD2/SMAD3 complexes inhibit proliferation. In certain cell types, JNK and p38 are phosphorylated by TAK1 and constitute, together with the PI3K-AKT-FOXO axis, ERK, and PAR6, so-called noncanonical signaling responses to TGF-β. The dashed line indicates unclear molecular mechanism.
TGF-β signaling pathways. TGF-β ligands bind type I and type II receptors at the cell surface. Subsequently, the type I receptor (ALK5) becomes phosphorylated by the type II receptor. This leads to phosphorylation of SMAD2 and SMAD3, which form a complex with SMAD4. Activated complexes accumulate in the nucleus where they cooperate with DNA-binding cofactors to regulate target gene transcription. SMAD2 and SMAD3 also bind to TIF1γ. In embryonic stem cells, SMAD2/3-TIF1γ recognizes specific chromatin marks, promoting access of SMAD2/3-SMAD4 to otherwise repressed targets. TIF1-γ–SMAD2/3 promotes erythroid differentiation whereas SMAD4-SMAD2/SMAD3 complexes inhibit proliferation. In certain cell types, JNK and p38 are phosphorylated by TAK1 and constitute, together with the PI3K-AKT-FOXO axis, ERK, and PAR6, so-called noncanonical signaling responses to TGF-β. The dashed line indicates unclear molecular mechanism.
Alternative pathways
The SMAD pathway forms a fundamental signaling module and is the most well-studied circuitry downstream of TGF-β. However, activation of other pathways, most notably TGF-β–activated kinase 1 (TAK1), a component of the mitogen-activated protein kinase pathway, has been observed in other cell types.8,9 Depending on context, TAK1 activates a host of downstream transducers, including p38 and c-Jun N-terminal kinase (JNK).10 Conditional deletion of TAK1 in mice, using the Mx-Cre driver, results in hematopoietic failure, but the link to TGF-β has not been firmly established.11 Other non-SMAD circuitries regulated by TGF-β include extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K)-AKT-FOXO, and RHO-like small guanosine triphosphatasess, though the contributions of most of these mechanisms are not well defined in HSCs (Figure 2).10 In addition, transcriptional intermediary factor-1γ (TIF1γ) partners with SMAD2/3 (see “Variations on TGF-β signaling: a role for TIF1-γ”).12 The TGF-β pathway is intimately linked with other signaling circuitries (for readers interested in crosstalk mechanisms, we refer to Nishita et al,13 Labbé et al,14 Itoh et al,15 Emmrich et al,16 and Chabanon et al17 ).
TGF-β: inducer of quiescence
TGF-β is cataloged as one of the most potent inhibitors of HSC growth in vitro; a variety of culture systems support this notion.18-21 During homeostatic conditions, the majority of HSCs are in a quiescent cell-cycle state.22,23 Quiescence is related to maintenance of the HSC pool and loss thereof results in exhaustion and erosion of HSC function.24 Naturally, TGF-β has been hypothesized to be a cardinal regulator of HSC dormancy, maintaining a pool of quiescent HSCs in vivo. Indeed, neutralization of TGF-β in vitro releases early hematopoietic progenitor cells from quiescence.25-27 Several molecular mechanisms have been proposed to account for TGF-β–mediated growth inhibition, including alterations in cytokine receptor expression and upregulation of cyclin-dependent kinase inhibitors, such as p15Ink4b, p21Cip1, and p27Kip1.26,28-34 However, TGF-β can exert growth inhibitory actions independent of p21Cip1 and p27Kip1.35 In human CD34+ cells, TGF-β–mediated cell-cycle arrest occurs through upregulation of p57Kip2, another member of the cyclin-dependent kinase inhibitor family.36 Similarly, p57Kip2 is highly enriched in mouse CD34−Kit+Lin−Sca1+ (KLS) cells as opposed to the more mature and actively cycling CD34+KLS fraction.37 Interestingly, a high level of p57Kip2 correlates with the activation status of SMAD2/3, which are uniquely phosphorylated in freshly isolated CD34−KLS cells but not in CD34+KLS progenitors.38 Additionally, TGF-β upregulates p57Kip2 in CD34−KLS cells in vitro.38 These findings point to a mechanism where TGF-β functions to induce p57Kip2 within the most primitive HSC compartment, thus promoting their quiescent state in vivo (Figure 3).
Bidirectional effects of TGF-β. Low concentrations of TGF-β stimulate proliferation of My-HSCs, whereas Ly-HSCs are growth inhibited. A higher dose of TGF-β inhibits proliferation, irrespective of HSC subtype, and induces quiescence via SMAD2/3-SMAD4-dependent expression of p57Kip2.
Bidirectional effects of TGF-β. Low concentrations of TGF-β stimulate proliferation of My-HSCs, whereas Ly-HSCs are growth inhibited. A higher dose of TGF-β inhibits proliferation, irrespective of HSC subtype, and induces quiescence via SMAD2/3-SMAD4-dependent expression of p57Kip2.
Heterogeneity of the HSC pool
Recently, evidence has accumulated suggesting that the adult HSC compartment consists of a number of functionally distinct subsets with diverse self-renewal and differentiation potentials.23,39-41 Interestingly, discrete HSC subtypes respond differently to TGF-β, according to a model proposed by Challen and colleagues.42 Specifically, TGF-β stimulates proliferation of myeloid-biased HSCs (My-HSCs) whereas lymphoid-biased HSCs (Ly-HSCs) are growth inhibited (Figure 3).42 Thus, the effect of TGF-β on HSCs is more nuanced than previously thought, such that proliferation is induced in certain HSC subtypes at certain concentrations. However, though TGF-β represents a potential signal for differential regulation between HSC subtypes, there is currently no tangible explanation for the underlying molecular mechanism. Another study found that the RNA-binding protein MUSASHI-2 is required for HSCs to mount a proliferative response to TGF-β at low concentrations.43 Loss of MUSASHI-2 leads to impaired HSC quiescence, reduced My-HSC numbers, and myeloid output in vivo. These findings are coupled to significant reductions in p57Kip2 and phosphorylated SMAD2/3 in HSCs, suggesting that MUSASHI-2 modulates the TGF-β signaling circuitry.43 Most work regarding TGF-β in hematopoiesis has focused on TGF-β1. However, TGF-β exists in 3 isoforms that are encoded by separate genes, Tgfb1-3. Although TGF-β1-3 share significant sequence homology and signal through the same receptor complex,44,45 differences in receptor affinity exist and varying responses have been reported. Most notably, KLS cells exhibit a biphasic response to TGF-β2, being growth inhibited at high doses and stimulated at low concentrations.46 The bidirectional effects of TGF-β on proliferation vs quiescence further substantiate the complexity of this signaling pathway, and it remains to be clarified whether the SMAD pathway is differentially regulated at high and low doses and between diverse HSC subtypes. Additionally, it is conceivable that the bidirectional effects are a function of contrasting TGF-β receptor expression among HSC subtypes, translating to dosage effects that generate qualitatively different responses. This theory is corroborated by a recent study, which shows that a high level of ALK5 correlates with increased myeloid output.47
TGF-β and the aging hematopoietic system
The hematopoietic system declines functionally with age, a phenomenon that can be traced back to alterations in the HSC compartment. The clonal composition of the HSC pool changes over time, such that My-HSCs become more abundant at the expense of Ly-HSCs.48 Physiologically, this translates to a myeloid-biased hematopoietic system with reduced lymphoid potential. Furthermore, the risk of hematologic malignancies, anemia, autoimmunity, and inflammatory disorders increases with age.48 As TGF-β differentially regulates My- and Ly-HSCs, the connection to aging is evident, especially because TGF-β is implicated in the aging process of nonhematopoietic tissues.49,50 A recent study (which profiled the transcriptome, DNA methylome, and histone modifications in highly purified young and old HSCs) found significant changes in the TGF-β pathway at the transcriptional level.51 In fact, the TGF-β pathway represented 19% of differentially expressed genes in young vs old HSCs. The study shows changes in multiple layers of the TGF-β pathway, from extracellular regulators, to SMAD transcription factors, and cofactors, resulting in an overall reduction of TGF-β signaling in aged HSCs.51 Because low concentrations of TGF-β stimulate proliferation of My-HSCs in vitro, downsizing the TGF-β circuitry may provide a more proliferative environment for My-HSCs, which can expand over time. Similarly, the stimulatory effect of TGF-β2 on the much cruder KLS population increases with age.52 This is logical, as the aged KLS compartment should contain a larger proportion of My-HSCs. However, the relationship between TGF-β and aging is complex, as evidenced by a study of TIF1-γ. In HSCs, the expression of TIF1-γ decreases with age, consistent with an accelerated aging phenotype of TIF1-γ knockout mice.47 Additionally, TIF1-γ controls the turnover of ALK5, such that ALK5+ HSCs become more abundant with age.47 Interestingly, aged HSCs are more sensitive to the cytostatic effect of TGF-β in vivo, possibly as a reflection of more generous ALK5 expression. As TIF1-γ is itself part of the signal transduction machinery downstream of TGF-β, the balance between TIF1-γ and SMAD4-mediated transcriptional responses may play an additional role, which was not investigated. In sum, perturbations of TGF-β signaling represent a potential mechanism that contributes to HSC aging.
TGF-β and the BM niche
The quest to decipher the role of TGF-β in the regulation of HSCs requires an understanding of the BM niches that house HSCs. Due to the anatomical complexity of the BM, much of our knowledge concerning the HSC microenvironment has for long remained nebulous and it is only in the last decade that we have gained a more detailed appreciation of the regulatory elements of the BM environment, although Ray Schofield proposed the concept of the BM niche already as far back as 1978.53 An increasing number of niche components have now been identified, revealing a complex network of cell-cell interactions, extracellular elements, signaling cues, and structures.54,55 Together, these entities create specialized niches that function to maintain and control self-renewing HSCs in a coordinated manner. TGF-β is part of this microenvironment, as it is produced by a variety of cell types in the BM, and large quantities of latent TGF-β are deposited into bone matrix.56,57 However, according to 1 study, surprisingly few BM cells exhibit significant phosphorylation of SMAD2/3 at steady state, suggesting that activation of latent TGF-β is tightly regulated spatially.58 Nonmyelinating Schwann cells, which ensheath peripheral nerves and lay in parallel with blood vessels in the BM, are proposed as one of the major sources for TGF-β activation in the BM.58 These glial cells regulate the activation process of TGF-β by binding latent TGF-β via integrin β8 on the cell surface, thus promoting activation by exposing TGF-β to proteolytic cleavage by metalloproteinases.58 A sizable portion of HSCs is in direct contact with nonmyelinating Schwann cells in the BM, and denervation results in loss of HSCs and increased cell cycling. Another major source of TGF-β is megakaryocytes, a cell type that physically associates with ∼20% of HSCs in the BM.59,60 HSCs in close proximity to megakaryocytes exhibit activation of SMAD2/3, and ablation of megakaryocytes results in reduced phosphorylation of SMAD2/3 as well as loss of quiescence and increased HSC proliferation.60 Importantly, conditional deletion of Tgfb1 in megakaryocytes results in increased cell cycling of HSCs.60 The anatomical relationship between megakaryocytes and Schwann cells has not been investigated, but the conclusion from these studies is that, indeed, TGF-β provides an important quiescence signal to HSCs in the BM niche. Additionally, the conclusion must be that >1 cell type in the BM contributes to TGF-β production and signaling in HSCs (Figure 4).
TGF-β in the BM niche. HSCs reside in the BM, in specialized niches that support and regulate HSC fate options. TGF-β is activated by Schwann cells that ensheath sympathetic nerves. Large amounts of TGF-β are also produced by megakaryocytes. HSCs in close proximity to Schwann cells and megakaryocytes become exposed to TGF-β and exhibit activation of SMAD2/3.
TGF-β in the BM niche. HSCs reside in the BM, in specialized niches that support and regulate HSC fate options. TGF-β is activated by Schwann cells that ensheath sympathetic nerves. Large amounts of TGF-β are also produced by megakaryocytes. HSCs in close proximity to Schwann cells and megakaryocytes become exposed to TGF-β and exhibit activation of SMAD2/3.
Lessons from in vivo models
TGF-β can affect most cell types throughout the hematopoietic hierarchy, but the response is modulated by context and differentiation stage of the target cell. Therefore, TGF-β generates highly variable biological outcomes and can affect proliferation, differentiation, and apoptosis in both positive and negative directions.21,61-63 Much of our knowledge of the physiological relevance of TGF-β has been gained by studying knockout mouse models. Based on these reports, we know that TGF-β is a principal regulator of immune cell homeostasis and function in vivo. Importantly, both Tgfb1-ligand and receptor knockout mice develop a lethal inflammatory disorder.64-66 With respect to hematopoiesis, Tgfb1-null mice exhibit enhanced myelopoiesis, suggesting that TGF-β acts as a negative regulator of myelopoiesis in vivo.65 Upon analysis before the onset of inflammation, a host of HSC properties are altered in Tgfb1 knockout mice.67 Most significantly, BM cells from Tgfb1-deficient neonates exhibit impaired reconstitution ability upon transplantation, a finding attributed to defective homing.67 However, mice deficient in ALK5 display normal HSC self-renewal and regenerative capacity in vivo, even under extreme hematopoietic stress with no defects in homing capacity.68,69 In contrast, TβRII conditional knockout mice show increased HSC cell cycling in vivo, and reduced regenerative capacity upon transplantation.58 Because of the severe inflammatory disease that develops in TGF-β signaling–deficient mouse models, most studies have used different strategies to overcome disease progression, including a variety of immune-deficient genetic backgrounds. This may account for the differences observed between knockout models. Additionally, signals may emanate from TβRII independently of ALK5, as has been shown in nonhematopoietic cell types where the cell polarity regulator partitioning defective 6 (PAR6) is phosphorylated directly by TβRII.70,71 Furthermore, the fact that TβRII is more highly expressed within HSCs compared with ALK5 might be an additional contributing factor.57 Thus, there are both overlapping and nonoverlapping phenotypes between knockout models and it appears to be critically important at which level and for how long TGF-β signaling is disrupted. This notion is supported by a recent study in which a neutralizing antibody against active TGF-β was administered to mice following 5-fluorouracil treatment.72 TGF-β blockade after chemotherapy results in enhanced hematopoietic regeneration by delaying the return of HSCs to quiescence. These findings imply that the TGF-β–SMAD2–p57Kip2 signaling axis is important for reestablishing quiescence of HSCs following stress, once sufficient regeneration of the hematopoietic system has been attained. Additionally, overexpression of SMAD4 sensitizes human cord blood–derived candidate HSCs to TGF-β, resulting in reduced regenerative capacity in vivo.73 Together, these findings suggest that transient inhibition of TGF-β might be a feasible strategy to enhance hematopoietic recovery in patients following chemotherapy. Due to the multifaceted nature of TGF-β coupled with a potentially complex set of redundant mechanisms, its role as a critical regulator of HSC quiescence in vivo has been difficult to unveil. However, current dogma now suggests that TGF-β is indeed an important quiescence signal in vivo.
A role for SMAD signaling in self-renewal of HSCs
To block the SMAD signaling network downstream of TGF-β, 2 approaches have been used: overexpression of the inhibitory SMAD7 and deletion of Smad4. In murine HSCs, forced expression of SMAD7 results in increased self-renewal of HSCs, indicating that the SMAD pathway negatively regulates self-renewal in vivo.74 Importantly, differentiation was unperturbed in this model, suggesting that self-renewal is regulated independently of differentiation by SMAD signaling. In contrast, overexpression of SMAD7 in human severe combined immunodeficiency repopulating cells results in altered differentiation from lymphoid-dominant engraftment toward increased myeloid contribution.75 Thus, in the xenograft model system, forced expression of SMAD7 modulates differentiation of multipotent human severe combined immunodeficiency repopulating cells. Using a conditional knockout mouse model, disruption of the SMAD pathway at the level of SMAD4 was investigated. Intriguingly, Smad4-deficient HSCs display a reduced repopulative capacity of primary and secondary recipients, indicating that SMAD4 is critical for HSC self-renewal in vivo.76 Because overexpression of SMAD7 vs deletion of Smad4 is anticipated to yield similar hematopoietic phenotypes, it is conceivable that SMAD4 functions as a positive regulator of self-renewal independently of its role in the TGF-β pathway.13-15 Alternatively, SMAD7 may have unanticipated functions in an overexpression setting that impinge positively on self-renewal.
Variations on TGF-β signaling: a role for TIF1γ
SMAD4 has traditionally been viewed as the nexus of SMAD signaling as it functions as a core component of both TGF-β/activin and BMP signaling branches. However, SMAD2/3 can also partner with TIF1γ.12 In human hematopoietic stem/progenitor cells, TGF-β balances erythroid differentiation with growth inhibition in a mechanism dependent on competitive binding between SMAD4 and TIF1γ to SMAD2/3.12 In response to TGF-β, the TIF1γ-SMAD2/3 complex stimulates erythroid differentiation whereas SMAD2/3 in association with SMAD4 leads to growth inhibition of human hematopoietic progenitors (Figure 2). Interestingly, the zebrafish homolog of TIF1γ, encoded by moonshine, is essential for blood formation with mutants displaying severe red cell aplasia, indicating that TIF1γ is required for erythroid development.77 Furthermore, TIF1γ is implicated in regulation of transcription elongation of erythroid genes. The proposed model suggests that TIF1γ functions to release paused Pol II at erythroid genes by recruiting positive elongation factors to the blood-specific transcriptional complex, thus promoting transcription.78 TIF1γ also functions at the erythroid/myeloid lineage bifurcation by modulating GATA1 and PU.1 expression.79 The link between TIF1γ and TGF-β signaling has been delineated in detail in embryonic stem cells. There, TIF1γ functions as a chromatin reader where TIF1γ-SMAD2/3 recognizes certain repressive histone marks, promoting a transition to open chromatin that allows SMAD4-SMAD2/3 to gain access to DNA.80 Thus, TIF1γ is needed for certain SMAD4-SMAD2/3 transcriptional responses.
TGF-β in hematopoietic disease
Leukemia
Despite TGF-β’s pronounced cytostatic effect on HSCs in vitro and the fact that mutations in genes encoding components of the TGF-β pathway are frequently found in other neoplasms, such as pancreatic and colon cancer,81,82 inactivating mutations are relatively uncommon for this pathway in hematologic malignancies.83 Nevertheless, a number of cases have been reported involving SMAD4 and TGFBR2 in patients with acute myelogenous leukemia (AML).84-87 For example, in a study investigating human AML genomes, various copy number alterations were found recurrently modified, 1 of which represented the deletion of SMAD4.88 Furthermore, reports of sporadic mutations in both TGFBR1 and TGFBR2 in lymphoid neoplasms exist, and loss of SMAD3 is associated with T-cell acute lymphocytic leukemia.89-91 The mechanism for the SMAD3 deficiency is not known because the SMAD3 messenger RNA was present and no mutations could be detected in the MADH3 gene, which encodes SMAD3.91 However, T-cell leukemogenesis is promoted in mice with haploinsufficiency of Smad3 and a complete deficiency of p27Kip1.91 These findings are interesting because the p27Kip1 gene is frequently mutated in pediatric acute lymphocytic leukemia, due to translocations and deletions or germline mutations.92,93 Impaired TGF-β signaling in hematologic malignancies can also be caused by suppression of SMAD-dependent transcriptional responses by oncoproteins like TAX, EVI-1, and AML1-ETO.94-96 Similarly, downregulation of the transcription factor ZEB1 and overexpression of SMAD7 contribute to resistance to TGF-β1–mediated growth suppression in adult T-cell leukemia/lymphoma without known mutations in TGF-β pathway genes.97 In addition, there are several reports on oncoproteins, which generate leukemia and simultaneously neutralize the growth inhibitory signal of the SMAD pathway by binding to or interacting with SMADs. Fusion oncoproteins, like TEL-AML1 and AML1-EVI1, bind to SMAD3, impairing both TGF-β signaling and apoptosis of transduced HSCs in vitro.95,98-100 Furthermore, SMAD4 physically associates with HOXA9, reducing its ability to regulate transcriptional targets in hematopoietic cells in vitro.101 In vivo studies show that in wild-type mice overexpressing HOXA9 or NUP98-HOXA9, SMAD4 binds the oncoproteins and sequestrates them to the cytoplasm, suggesting that SMAD4 plays a protective role against further promotion and growth of leukemic cells.102 Therefore, SMAD signaling can be reduced or neutralized in hematopoietic malignancies, but in a majority of cases this is not due to mutations in genes of the circuitry itself, but rather through altered expression or function of cofactors and oncoproteins, or alternatively via loss of TGF-β target genes.
The diseased BM microenvironment
Although TGF-β plays a major role as tumor suppressor, TGF-β can paradoxically facilitate tumor growth, particularly in the later stages of disease. This is due to effects on the tumor microenvironment and the immunosuppressive function of TGF-β. Interestingly, recent findings show that there is considerable interplay between leukemic cells and the BM microenvironment. In fact, the BM microenvironment undergoes substantial remodeling by signals from leukemic cells.103 Similarly, alterations in niche cells can trigger hematopoietic disease and promote leukemia.104,105 Furthermore, activation of the parathyroid hormone receptor specifically in osteoblastic cells results in remodeling of the BM microenvironment.106 The remodeled niche attenuates BCR-ABL1 oncogene-induced chronic myeloid leukemia–like disease, via upregulation of TGF-β1, whereas MLL-AF9 oncogene-induced AML is enhanced. These data differ from the findings by Naka et al, in which TGF-β, via regulation of AKT and FOXO3a, instead maintains leukemia-initiating cells in chronic myeloid leukemia.107 The discrepancies may be attributed to differences in TGF-β dosage and context, indicating that TGF-β sensitivity across different types of leukemia varies. Nevertheless, the findings point to the fact that TGF-β is part of the leukemic BM niche and strategies to target the BM microenvironment may be a promising approach to reduce certain types of leukemic stem cells in the future. On this note, TGF-β has been proposed to contribute to myelofibrosis, which is a chronic myeloproliferative disease characterized by clonal proliferation and BM fibrosis.108 Several cytokines are thought to contribute toward accumulation of reticulin fibers in the BM of patients with myelofibrosis. These include TGF-β, fibroblast growth factor 2 (FGF-2), and platelet-derived growth factor.109-112 Some of the most compelling evidence for a prominent role of TGF-β in myelofibrosis comes from a study using Tgfb1-null mice. To induce myelofibrosis, irradiated mice were transplanted with BM cells transduced with vectors containing the thrombopoietin gene. Importantly, prominent myelofibrosis developed in mice receiving transduced wild-type cells but not Tgfb1-null cells.111 These data indicate that TGF-β1, produced by hematopoietic cells, is a crucial component in the development of myelofibrosis, and underlines further the interplay between hematopoietic cells and the BM microenvironment.
TGF-β in the pathogenesis of MDS
Myelodysplastic syndromes (MDSs) encompass a spectrum of clonal stem cell disorders characterized by inefficient hematopoiesis and reduced peripheral blood counts. Excessive activation of inhibitory pathways, such as TGF-β, has been proposed to amplify the inefficient blood production inherent to MDS.113 For example, SMAD2 is upregulated and overactivated in CD34+ BM progenitors from MDS patients. Importantly, pharmacologic inhibition of the TGF-β pathway in vivo, using a small-molecule inhibitor of ALK5, alleviates anemia in a mouse model of MDS.114 Furthermore, SMAD7 is reduced in BM progenitors from MDS patients, leading to overactivation of TGF-β signaling.115 The reduction of SMAD7 is attributed to increased levels of microRNA-21, which binds to the 3′ untranslated region of SMAD7.116 Administration of a chemically modified inhibitor of microRNA-21 results in increased red blood cell counts, using the same MDS mouse model mentioned in “The diseased BM microenvironment.”116 Together, these studies implicate TGF-β in the pathogenesis of MDS and suggest that this pathway may be a realistic therapeutic target in a subset of MDSs.
Therapeutic avenues and future perspectives
HSCs are the therapeutic units of BM transplantations and, as such, are by far the most widely used application of stem cell therapy to date. Despite this, strategies to improve efficiency and applicability of HSC transplantations are needed, including expansion of HSCs ex vivo and techniques to improve engraftment and regeneration posttransplantation. Although BMPs have been reported to contribute to HSC expansion ex vivo, manipulating SMAD signaling alone is unlikely to result in effective expansion.117 Another approach, with substantial promise that should be investigated further, is to transiently block TGF-β signaling in vivo to boost regeneration and recovery of hematopoiesis following chemotherapy.72 Similarly, a number of recent studies highlight the feasibility of manipulating the TGF-β pathway in hematopoietic disorders like MDS and anemia.115,118,119 In addition, the complexity of the SMAD pathway continues to be exposed as new layers of regulatory mechanisms are found. For example, the linker region of SMADs is subject to negative regulation by glycogen synthase kinase 3, FGF, and epidermal growth factor.120-123 The importance of this type of crosstalk in hematopoietic cells is unknown, but it is worth mentioning as megakaryocytes were recently shown to balance TGF-β and FGF output, thereby regulating hematopoietic regeneration following 5-fluorouracil challenge in vivo.60 Thus, manipulating TGF-β signaling is relevant to several aspects of hematopoiesis. In the future, more detailed mechanistic studies are required to precisely define how TGF-β signaling may be manipulated in relation to other signaling circuitries, ultimately improving HSC regeneration and hematopoietic disease in vivo.
Acknowledgments
The authors apologize to those whose work was not cited due to space limitations.
This work was supported by the European Commission (Stemexpand); Hemato-Linné and Stemtherapy program project grants from the Swedish Research Council; a project grant from the Swedish Research Council (S.K.); the Swedish Cancer Society; the Swedish Children Cancer Foundation; a Clinical Research Award from Lund University Hospital; and a grant from The Tobias Foundation awarded by the Royal Academy of Sciences (S.K.).
Authorship
Contribution: U.B. and S.K. wrote the review, and U.B. designed the figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan Karlsson, Division of Molecular Medicine and Gene Therapy, BMC A12, 221 84 Lund, Sweden; e-mail: Stefan.karlsson@med.lu.se; and Ulrika Blank, Division of Molecular Medicine and Gene Therapy, BMC A12, 221 84 Lund, Sweden; e-mail: ulrika.blank@med.lu.se.