Key Points
A significant fraction of cell-free heme in plasma is contained in circulating erythrocyte MPs, particularly during hemolysis.
Heme-laden MPs can transfer heme to vascular endothelium and mediate oxidative stress, vascular dysfunction, and vasoocclusions.
Abstract
Intravascular hemolysis describes the relocalization of heme and hemoglobin (Hb) from erythrocytes to plasma. We investigated the concept that erythrocyte membrane microparticles (MPs) concentrate cell-free heme in human hemolytic diseases, and that heme-laden MPs have a physiopathological impact. Up to one-third of cell-free heme in plasma from 47 patients with sickle cell disease (SCD) was sequestered in circulating MPs. Erythrocyte vesiculation in vitro produced MPs loaded with heme. In silico analysis predicted that externalized phosphatidylserine (PS) in MPs may associate with and help retain heme at the cell surface. Immunohistology identified Hb-laden MPs adherent to capillary endothelium in kidney biopsies from hyperalbuminuric SCD patients. In addition, heme-laden erythrocyte MPs adhered and transferred heme to cultured endothelial cells, inducing oxidative stress and apoptosis. In transgenic SAD mice, infusion of heme-laden MPs triggered rapid vasoocclusions in kidneys and compromised microvascular dilation ex vivo. These vascular effects were largely blocked by heme-scavenging hemopexin and by the PS antagonist annexin-a5, in vitro and in vivo. Adversely remodeled MPs carrying heme may thus be a source of oxidant stress for the endothelium, linking hemolysis to vascular injury. This pathway might provide new targets for the therapeutic preservation of vascular function in SCD.
Introduction
Sickle cell disease (SCD) is a single gene disorder characterized by mutant hemoglobin-S, causing a wide variety of symptoms, including chronic intravascular hemolysis, high plasma levels of cell-free heme and hemoglobin (Hb),1-5 and vascular dysfunction. During hemolysis, iron ions, heme, and Hb exert potent oxidative reactions deleterious for blood vessels. Physiological protection against cell-free heme and Hb is provided by circulating hemopexin (Hpx) and haptoglobin, which mediate heme clearance by macrophages and hepatocytes.2,6-9 This scavenging system is overwhelmed by hemolysis in SCD, leading to the accumulation of plasma Hb and heme,2-4 exacerbating vascular injury.
Vascular dysfunction in SCD is materialized by chronic ischemic injury to multiple organs, including kidneys.5,10,11 Chronic ischemia is associated with painful vasoocclusive crises (VOCs), precipitated by inadequate vasodilation, erythrocyte trapping and hemolysis.5,12,13 The mechanisms of vascular injury in SCD are debated and may include chronic overproduction of endothelin-114 and reduced nitric oxide (NO) bioavailability because of its scavenging by cell-free Hb.15-17 Vascular injury involves decreased vasodilation, erythrocyte trapping, and painful VOCs associated with a further rise in hemolysis.5,12,13 Endothelial injury may also trigger chronic degenerative manifestations through ischemic events and functional vascular remodeling observed in mice18-20 and humans.10,11,21-24
Cell-free Hb and erythrocyte microparticle (MP) levels were recently found to correlate with hemolysis, oxygen saturation, pulmonary systolic pressure, and mortality in SCD patients.13 In mice, Hb and heme may contribute to vascular injury and blood stasis via the activation of Toll-like receptor-4 (TLR-4), nuclear factor κB signaling, von Willebrand factor release in endothelial cells,25,26 and the release of DNA by neutrophils.27
Little is known about the presentation of cell-free heme and Hb to endothelial cells. In hemolytic disorders, plasma is rich in submicron membrane vesicles called MPs, which express phosphatidylserine (PS).28 MP levels are 3- to 10-fold higher in SCD patients during steady state than in healthy control subjects under steady-state conditions,4,29-31 and further increased 0.3- to 3-fold during VOC.32-34 SCD MPs are mostly of red blood cell (RBC) origin4,30-33 and may be produced through accelerated aging of sickle cells during oxygenation/deoxygenation cycles,28 under the influence of stress factors35,36 and matrix proteins,20 or during hemolysis in capillary beds.
We recently described the specific ability of RBC MPs to induce vasoocclusions in a mouse model of SCD, with reduced renal perfusion and microvascular clogging.20 Concurrently, Donadee and colleagues showed that artificial aging of erythrocytes in blood storage bags released MPs containing Hb that could alter vascular function in rats by reducing NO bioavailability.37
In this work, we show that MPs from human SCD plasma or generated in vitro from RBCs contain heme. We found that heme-laden RBC MPs closely associated with renal capillary endothelium in human and mouse SCD biopsies and blocked flow-mediated vasodilation when perfused into mouse mesenteric arteries. In vitro, we show that heme-laden RBC MPs induce oxidative stress and apoptosis in endothelial monolayers. These toxic properties were all reversed when MPs were incubated with the PS antagonist annexin-a5 or with Hpx, establishing the role of heme in the vascular insult mediated by RBC MPs in SCD.
Materials and methods
For more information on materials and methods, see the supplemental Methods (available on the Blood Web site).
Human subjects and the HEMIR cohort
Forty-seven SCD patients with hemoglobin SS (HbSS; and under 15% patients with HbSβ0 thalassemia) and 22 matched healthy volunteers of 18 years of age or more were enrolled after informed consent and approval by the ethical committees of the French Scientific Research Ministry; collection protocol DC-2011-1450–HEMIR (heme, microparticles and red blood cells) (supplemental Table 1). Research was conducted in accordance with the Declaration of Helsinki and included (1) patients with HbSS in steady state and (2) healthy African-descent control subjects matched in sex, age, and ethnics distribution. Patients under hydroxycarbamide (hydroxyurea) or in a chronic transfusion program were excluded.
MP generation and characterization
Concentrated MP stocks were generated from isolated phosphate-buffered saline (PBS)–washed erythrocytes as described.20 Supernatant MPs were ultracentrifuged (20 500 g, 45 minutes), resuspended in 0.2 μm filtered PBS, aliquoted, and stored at −80°C (freeze-thawed once only). PS+ MPs were quantified as previously described20,38 with fluorescein isothiocyanate–conjugated annexin-V (Roche Diagnostics, France) and CaCl2 on a Coulter EPICS XL flow cytometer (Beckman Coulter), as events of 0.2 to 1 μm in diameter (supplemental Figure 1). Annexin-V-labeled MP concentrations were determined with respect to calibrated fluorescent microbeads (Flowcount; Beckman Coulter).
Murine microvascular dilation
Dissected mouse mesenteric arteries were placed in ice-cold physiological salt solution (PSS). Four to six arterial segments from each mouse were cannulated and mounted in a video-monitored perfusion system as described.39 Cumulative concentration-response curves to acetylcholine (ACH; 10−7 to 10−4 M) were constructed after phenylephrine-preconstriction, at intraluminal pressure of 75 mm Hg and 20 µL/min flow.20
Murine model of SCD and kidney vasoocclusions
We used 10- to 18-week-old SAD transgenic male mice of C57bl6J background initially obtained from Dr Beuzard,40 carrying a human Hb β-chain transgene with 3 mutations (βS β6Val, βS-Antilles, β23Ile, and D-Punjab β121Glu). Purified MPs were injected intravenously.20 Renal vasoocclusions were identified by reduced kidney perfusion (blood flow velocity in renal arteries) measured by Echo-Doppler,14,20,41 using a Vivid 7 echograph (GE Medical Systems, Horten, Norway).
Results
Intravascular hemolysis, cell-free Hb, and erythrocyte MPs in SCD
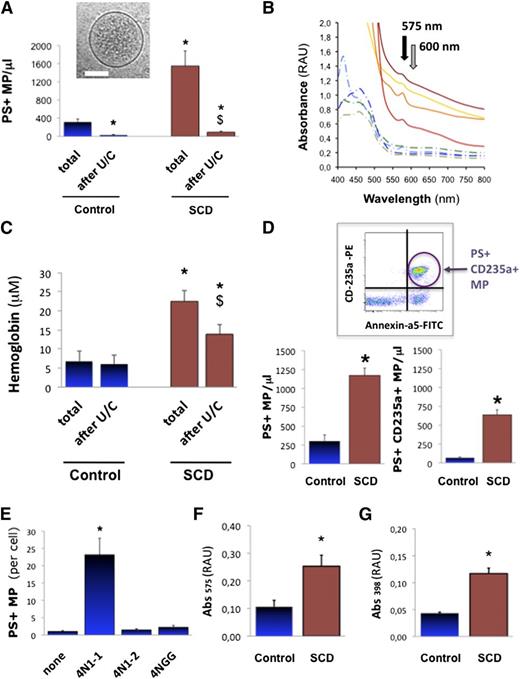
We first aimed at describing MPs in human SCD plasma with respect to hemolysis, Hb, and heme. We measured MPs in the plasma of 47 SCD patients (HbSS homozygous) and 22 healthy control age-, gender-, and ethnics-matched volunteers (supplemental Table 1). We found fivefold more annexin-a5+ MPs in SCD vs controls (Figure 1A; 1551 vs 314 MPs/μL; P < .001; supplemental Figure 1). Plasma Hb measured by Abs575 spectrophotometry (Figure 1B; supplemental Methods)37 reached 6.8 μM in controls and 22.5 μM in SCD, a threefold increase (Figure 1C; P = .007). We quantified MPs of erythrocyte origin by FACS (n = 12). We found a ninefold rise in annexin-a5+/CD235a+ MPs in SCD plasma vs controls (Figure 1D; 637 vs 67 MPs/μL; P = .01). Erythrocyte MPs amounted to 55% total annexin-a5+ MPs vs 22% in control plasma (P < .05). This supports previous reports of increased cell-free heme in SCD plasma (4 to 30 μM) vs controls (0.2 to 2 μM), with largely predominant erythrocyte MPs.4,30-33
Heme-MP association in SCD plasma. Circulating PS+ MPs (A) were quantified in platelet-free plasma (PFP) from steady-state SCD patients (n = 47) or control matched healthy volunteers (n = 22) by fluorescence-activated cell sorter (FACS) after annexin-a5 labeling, as well as plasma Hb by spectrophotometry (Abs575-Abs600), before and after MP depletion by ultracentrifugation (U/C). (B) Whole spectrum analysis of light absorbance in SCD (full lines) and control PFP (dotted lines). Black arrow marks peak of Hb absorbance at 575 nm; gray arrow points at 600 nm. (C) Absorbance (Abs575-Abs600) in PFP from control volunteers and steady-state SCD patients. *P < .01 vs total in controls; $P < .05 vs total in SCD. (D) Circulating erythrocyte MPs were quantified in plasma from control and SCD patients (n = 12) by FACS after phycoerythrin-conjugated anti-CD235a+ immunoglobulin G (IgG) labeling; *P = .01. We generated stocks of MPs shed by purified erythrocytes in vitro. SCD and control erythrocytes were incubated in vitro with CD47 agonist peptide 4N1-1, truncated 4N1-2, or mutated 4NGG peptide (25 μM, 30 minutes). (E) Quantification of MPs released by control erythrocytes, by FACS after annexin-a5 labeling. Supernatants were ultracentrifuged to pellet MPs, which were resuspended at similar concentrations in PBS (500 MPs/μ). Spectrophotometric measurements relative to Hb (Abs575) (F) and heme (Abs398) (G) were gathered. *P < .05 vs control MPs.
Heme-MP association in SCD plasma. Circulating PS+ MPs (A) were quantified in platelet-free plasma (PFP) from steady-state SCD patients (n = 47) or control matched healthy volunteers (n = 22) by fluorescence-activated cell sorter (FACS) after annexin-a5 labeling, as well as plasma Hb by spectrophotometry (Abs575-Abs600), before and after MP depletion by ultracentrifugation (U/C). (B) Whole spectrum analysis of light absorbance in SCD (full lines) and control PFP (dotted lines). Black arrow marks peak of Hb absorbance at 575 nm; gray arrow points at 600 nm. (C) Absorbance (Abs575-Abs600) in PFP from control volunteers and steady-state SCD patients. *P < .01 vs total in controls; $P < .05 vs total in SCD. (D) Circulating erythrocyte MPs were quantified in plasma from control and SCD patients (n = 12) by FACS after phycoerythrin-conjugated anti-CD235a+ immunoglobulin G (IgG) labeling; *P = .01. We generated stocks of MPs shed by purified erythrocytes in vitro. SCD and control erythrocytes were incubated in vitro with CD47 agonist peptide 4N1-1, truncated 4N1-2, or mutated 4NGG peptide (25 μM, 30 minutes). (E) Quantification of MPs released by control erythrocytes, by FACS after annexin-a5 labeling. Supernatants were ultracentrifuged to pellet MPs, which were resuspended at similar concentrations in PBS (500 MPs/μ). Spectrophotometric measurements relative to Hb (Abs575) (F) and heme (Abs398) (G) were gathered. *P < .05 vs control MPs.
Cryogenic transmission electron microscopy confirmed the vesicular nature of the material pelleted out of plasma by ultracentrifugation (Figure 1A inset; supplemental Figure 2). FACS confirmed the near complete removal of MPs (Figure 1A; >80% in SCD; P < .01). Interestingly, it was accompanied by a 38% reduction in plasma Hb (Figure 1C; P = .042 in SCD). Plasma Hb carried by MPs could thus reach up to 0.6 μM in controls and up to 10 μM in SCD. We also depleted plasma MPs by 0.1 μm filtration (supplemental Figure 3A-B; n = 4, P = .026 vs SCD). Again, MP removal reduced plasma Hb by more than a third. Hence, significant amounts of cell-free Hb and heme may segregate to circulating MPs. A commercial kit confirmed that MP removal reduced plasma heme by ∼25% in controls and 30% in SCD (supplemental Figure 3C; n = 10, P = .006 in controls; P = .028 in SCD). Calculations indicate that MP-borne heme may reach up to 1 μM in controls and 2 μM in SCD. In other words, 50 MPs/μL might carry up to 14 nM heme in controls, and 150 nM heme in SCD. Furthermore, analysis of Hb associated to plasma MPs pellets revealed that 20% to 35% consisted of methemoglobin (met-Hb) (supplemental Figure 4; n = 3 per group).
Erythrocyte vesiculation in vitro
We aimed to generate and describe stocks of purified erythrocyte MPs that could be manipulated in cultures and in vivo. To induce vesiculation, we stimulated freshly sorted human SCD and control erythrocytes with the CD47 agonist peptide 4N1-1, a peptide derived from the thrombospondin-1 carboxyterminus.20,42 Cryo-transmission electron microscopy on supernatants revealed that erythrocytes released MPs with a membrane and a size distribution compatible with native plasma. Erythrocytes released ∼12-fold more MPs when treated with 4N1-1 vs control peptides (Figure 1E; n = 4, P < .001). Spectrophotometry (Figure 1F-G; n = 3) determined that MPs released by SCD erythrocytes contained twice more heme than control MPs (P = .038). In short, 50 control erythrocyte MPs/μL carried ∼20 nM heme, whereas 50 SCD erythrocyte MPs/μL carried ∼65 nM heme. Met-Hb represented a large part (45% to 75%) of Hb associated to erythrocyte MP (supplemental Figure 4). SCD MP displayed a trend of higher met-Hb contents, although not significant. We concluded that in vitro vesiculation concurred with Hb and heme release, resulting in met-Hb- and heme-laden MPs.
Phospholipid vesicles concentrate heme
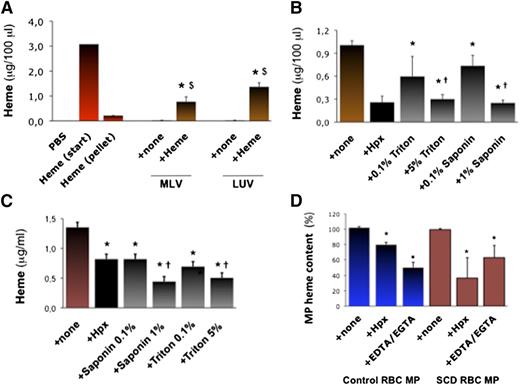
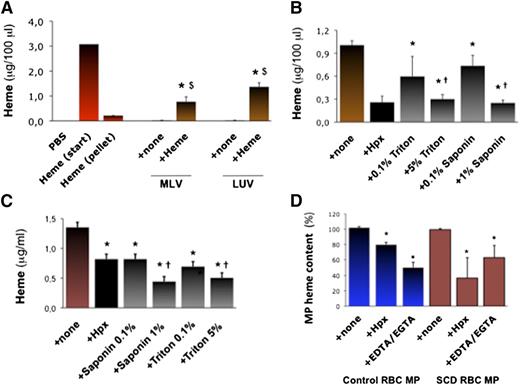
We aimed to generate synthetic phospholipid vesicles to mimic erythrocyte MPs. Multilayer vesicles (MLVs) or large unilamellar vesicles (LUVs) prepared in vitro by phospholipid extrusion contained no detectable heme (Abs398; Figure 2A). However, MLVs and LUVs incubated in heme absorbed it (∼10 μg heme for 50 000 vesicles; n = 3, P = .033 and 0.031). Conversely, Hpx (10 μM for 2 hours) strongly reduced heme incorporation into MLVs (Figure 2B; n = 3, P < .001), so did the detergents triton and saponin (0.1% to 5% ; P < .001 vs none).
Characterization of heme interactions with MP membranes. We synthetized MLVs or LUVs in vitro. (A) We incubated MLVs and LUVs (5000 vesicles/100 μL) in 50 μM heme or PBS (+ none) for 1 hour. Vesicles were then washed and concentrated in PBS. We quantified heme in vesicles alone and in heme-incubated vesicles by spectrophotometry (Abs398), compared with serial heme dilutions. Abs398 was also assessed in the initial heme solution before (start) and after ultracentrifugation without vesicles (pellet). Abs398 was measured in vesicles prior to or after incubation in heme. $P < .05 vs heme after ultracentrifugation (pellet); $P < .05 vs vesicles + none. (B) Heme-incubated MLVs were treated for 1 hour with triton and saponin (up to 5%) or Hpx (10 μM). Abs398 was then measured after de novo ulracentrifugation. *P < .01 vs MLV + none; †P < .05 vs lower detergent concentrations. (C) SCD erythrocyte MPs were incubated with triton or saponin (up to 5%), and Abs398 was measured compared with a heme dilution curve. *P < .05 vs MPs + none; †P < .05 vs low detergent concentrations. (D) In similar experiments, initial MP heme content was expressed at 100%. Control and SCD erythrocyte MPs (5000 PS+ MPs/100 μL; n = 4) were incubated with Hpx (2 μM) or EGTA-EDTA mix (both at 250 μM). *P < .05 vs MP + none.
Characterization of heme interactions with MP membranes. We synthetized MLVs or LUVs in vitro. (A) We incubated MLVs and LUVs (5000 vesicles/100 μL) in 50 μM heme or PBS (+ none) for 1 hour. Vesicles were then washed and concentrated in PBS. We quantified heme in vesicles alone and in heme-incubated vesicles by spectrophotometry (Abs398), compared with serial heme dilutions. Abs398 was also assessed in the initial heme solution before (start) and after ultracentrifugation without vesicles (pellet). Abs398 was measured in vesicles prior to or after incubation in heme. $P < .05 vs heme after ultracentrifugation (pellet); $P < .05 vs vesicles + none. (B) Heme-incubated MLVs were treated for 1 hour with triton and saponin (up to 5%) or Hpx (10 μM). Abs398 was then measured after de novo ulracentrifugation. *P < .01 vs MLV + none; †P < .05 vs lower detergent concentrations. (C) SCD erythrocyte MPs were incubated with triton or saponin (up to 5%), and Abs398 was measured compared with a heme dilution curve. *P < .05 vs MPs + none; †P < .05 vs low detergent concentrations. (D) In similar experiments, initial MP heme content was expressed at 100%. Control and SCD erythrocyte MPs (5000 PS+ MPs/100 μL; n = 4) were incubated with Hpx (2 μM) or EGTA-EDTA mix (both at 250 μM). *P < .05 vs MP + none.
Erythrocyte MPs generated in vitro (5000 PS+ MPs/100 μL) were also treated with Hpx, triton or saponin, or a mixture of EDTA and EGTA. MPs were ultracentrifuged and resuspended for a spectrophotometric evaluation of heme (Figure 2C). Triton (5%) and saponin (1%) reduced heme:MP association by up to 50% (n = 3, P < .05 for both). Interestingly, Hpx reduced heme:MP association by 20% in control MPs and 50% in SCD MPs (P = .022 and P = .035; Figure 2D). Removal of Ca2+ with EDTA/EGTA reduced heme by ∼50% in WT and SCD MPs (P = .077 and P = .040; Figure 2D). This led us to hypothesize that PS and Ca2+ might contribute to heme segregation in MPs.
In silico modeling of molecular interactions predicted that 1 heme molecule is capable of binding to 1 PS via 2 Ca2+ bonds. This is supported by calculation of interaction energies in a 3-dimensional representation, and by dynamic modeling of heme interacting with 1 PS embedded in a phosphatidylcholine and cholesterol membrane (supplemental Figure 5A-B).
Heme-laden MPs transfer heme to endothelial cells
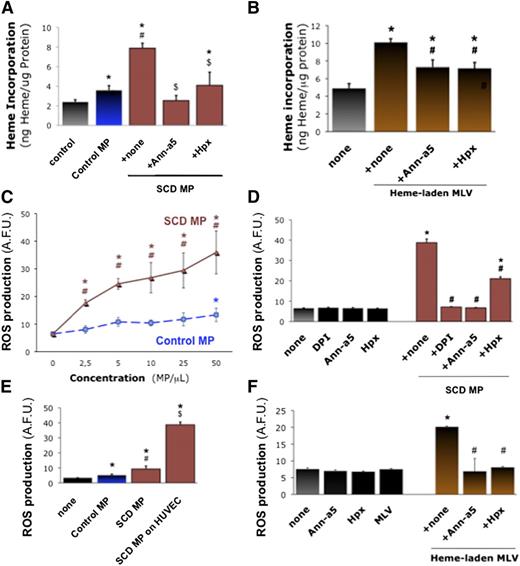
We investigated the interaction of heme-laden MPs with vascular endothelium as a possible target tissue to bind to and activate. We incubated cultured human umbilical cord vascular endothelial monolayers (HUVECs) with erythrocyte MPs (50 PS+ MPs/μL, 1 hour) and quantified heme transferred to HUVEC monolayers after washing (Figure 3A). Control erythrocyte MPs transferred heme to HUVECs (n = 6, P = .047 vs none), but MPs from SCD erythrocytes were fourfold more efficient (P = .014 vs none). Heme transfer was completely blocked by preincubating MPs with the PS antagonist annexin-a5 (10 μg/mL) or heme-scavenging Hpx (2 μΜ) for 1 hour (P = .032 and P = .042). We also placed synthetic heme-laden MLVs on HUVECs (50 MLVs/μL; Figure 3B). MLVs transferred heme to HUVECs (P < .001). Again, the incorporation of heme into HUVEC monolayers was sensitive to annexin-a5 and Hpx (n = 3, P < .05 vs MLVs alone).
Endothelial activation by heme-laden MPs. Confluent HUVEC monolayers were treated for 2 hours with erythrocyte MPs or synthetic vesicles. Some MPs were preincubated for 1 hour with specific inhibitors of MPs PS (10 μg/mL annexin-a5) and heme (Hpx, 2 μM). HUVECs were incubated with MPs derived from SCD or control erythrocytes (50 MPs/μL; ie, ∼20 nM heme for control MPs and 65 nM heme for SCD MPs) (A), or synthetic heme-laden MLVs (50 MLVs/μL) (B), washed with PBS, and lysed. Heme incorporation was estimated by spectrophotometry (Abs398) with respect to serial heme dilutions and normalized for protein contents; *P < .05 vs control; #P < .05 vs control MPs; $P < .05 vs MPs + none. (C) We analyzed ROS generation after incubation of HUVECs with SCD and control erythrocyte MPs (25 MPs/μL), with or without preincubation for 1 hour with annexin-a5 or Hpx. (D) HUVECs were preincubated for 1 hour with reduced NAD phosphate inhibitor DPI (10 μM). Here, fluorescent H2-DFF-DA was added after 30 minutes with MPs, and ROS production measured after 90 minutes. *P < .05 vs control; #P < .05 vs MPs + none. (E) Alternatively, we assessed ROS production by erythrocyte MPs alone for 1 hour in H2-DFF-DA, in absence of HUVECs. *P < .05 vs none; #P < .05 vs control MPs; $P < .05 vs SCD MPs without endothelium. (F) We assessed the effects of heme-laden MLVs on HUVEC ROS production, after incubation with annexin-a5 (10 μg/mL) or Hpx (2 μM). *P < .05 vs control; #P < .05 vs MLVs + none.
Endothelial activation by heme-laden MPs. Confluent HUVEC monolayers were treated for 2 hours with erythrocyte MPs or synthetic vesicles. Some MPs were preincubated for 1 hour with specific inhibitors of MPs PS (10 μg/mL annexin-a5) and heme (Hpx, 2 μM). HUVECs were incubated with MPs derived from SCD or control erythrocytes (50 MPs/μL; ie, ∼20 nM heme for control MPs and 65 nM heme for SCD MPs) (A), or synthetic heme-laden MLVs (50 MLVs/μL) (B), washed with PBS, and lysed. Heme incorporation was estimated by spectrophotometry (Abs398) with respect to serial heme dilutions and normalized for protein contents; *P < .05 vs control; #P < .05 vs control MPs; $P < .05 vs MPs + none. (C) We analyzed ROS generation after incubation of HUVECs with SCD and control erythrocyte MPs (25 MPs/μL), with or without preincubation for 1 hour with annexin-a5 or Hpx. (D) HUVECs were preincubated for 1 hour with reduced NAD phosphate inhibitor DPI (10 μM). Here, fluorescent H2-DFF-DA was added after 30 minutes with MPs, and ROS production measured after 90 minutes. *P < .05 vs control; #P < .05 vs MPs + none. (E) Alternatively, we assessed ROS production by erythrocyte MPs alone for 1 hour in H2-DFF-DA, in absence of HUVECs. *P < .05 vs none; #P < .05 vs control MPs; $P < .05 vs SCD MPs without endothelium. (F) We assessed the effects of heme-laden MLVs on HUVEC ROS production, after incubation with annexin-a5 (10 μg/mL) or Hpx (2 μM). *P < .05 vs control; #P < .05 vs MLVs + none.
Heme-laden MPs trigger endothelial ROS production and apoptosis
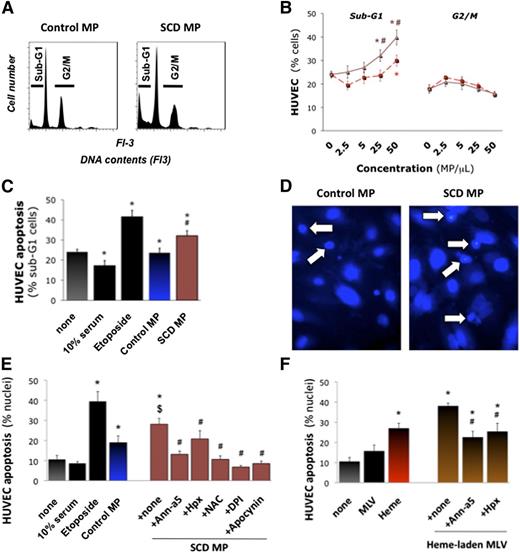
We asked whether the transfer of heme to endothelial cells by MPs may activate the cells. We quantified reactive oxygen species (ROS) production by cultured HUVECs incubated with erythrocyte MPs or MLVs for 1 hour, before adding 5-(and 6-)carboxy-2′,7′-difluorodihydrofluorescein diacetate (H2DFF-DA) for 30 minutes. SCD erythrocyte MPs triggered significant ROS production at 2.5 MPs/μL and above (Figure 3C; n = 4-8, P < .001 vs none or control MPs). Control MPs only induced moderate ROS production at 50 MPs/μL.
We preincubated SCD erythrocyte MPs with annexin-a5 or Hpx (Figure 3D), or HUVECs with reduced NAD phosphate oxidase inhibitors apocynin and diphenyleneiodonium (DPI) for 1 hour. DPI and annexin-a5 completely prevented ROS production, whereas Hpx reduced it by half (n = 4-8, P < .001 for all vs MPs alone). SCD and control MPs placed alone in H2DFF-DA for 1 hour only generated minimal amounts of ROS (Figure 3E). Synthetic heme-laden MLVs placed on HUVECs also stimulated ROS production vs MLVs alone (n = 3, P < .001), in an annexin-a5- and Hpx-sensitive fashion (Figure 3F).
We confirmed the production of ROS using Luminol (supplemental Figure 6A; n = 6, P < .001 vs control MPs) and by loading HUVECs with H2DFF-DA for 1 hour before washing and adding MPs (supplemental Figure 6B; n = 3, P < .001 vs none). Again, SCD MPs induced significant ROS accumulation, blocked by apocynin and DPI (10 μM), Hpx (2 μM), annexin-a5 (10 μg/mL), or ROS scavenger N-acetyl-L-cysteine (NAC) (5 mM) (P < .001 vs MPs alone). TLR-4 neutralization prevented ROS stimulation nearly entirely (supplemental Figure 6C; n = 3, P < .001 vs MP IgG).
SCD MPs at 25 and 50 MPs/μL increased apoptotic sub-G1 cells by 10% to 15% (Figure 4A; n = 6, P = .024 and P = .008 vs control MPs), whereas control MPs had little effect (Figure 4B-C). Fluorescence microscopy (Figure 4D) confirmed that SCD MPs doubled apoptotic nuclei (n = 9, P = .044 vs none; Figure 4E). Annexin-a5 and Hpx completely blocked these effects (P = .001 and P = .01 vs MPs alone). Moreover, heme-laden MLVs induced significant apoptosis vs MLVs alone (P < .001; Figure 4F).
Endothelial apoptosis by heme-laden MPs. Confluent HUVEC monolayers were treated with purified erythrocyte MPs (25 MPs/μL), synthetic heme-laden MLVs, or heme alone (5 μM) for 16 hours. Some MPs were preincubated with annexin-a5 (10 μg/mL) or Hpx (2 μM) for 1 hour. HUVECs were then fixed. Total DNA contents were determined by FACS after coloration with propidium iodide. Cells undergoing apoptosis (sub-G1 phase) or proliferation (G2/M) were quantified. (A) Representative DNA content profiles. We compared the effects of serial dilutions of control and SCD erythrocyte MPs (B) vs high serum (10% serum) or proapoptotic etoposide (100 μM). Triangles, SCD MPs; squares, control MPs. *P < .05 vs none; #P < .05 vs control MPs. (C) In other experiments, HUVECs were fixed in situ and stained with 4,6-diamidino-2-phenylindole. Nuclei displaying fragmentation, pyknosis, or condensed chromatin by fluorescence microscopy were counted as apoptotic and expressed as percentage. (D) Representative images (×400). Arrows designate fragmented and condensed nuclei. (E) Quantification of degraded nuclei after incubation with erythrocyte MPs (25 MPs/μL), vs high serum (10% serum), or proapoptotic etoposide. Some HUVECs were preincubated for 30 minutes with NAC (5 mM), DPI (10 μM), or apocynin (100 μM). *P < .05 vs none; $P < .05 vs control MPs; #P < .05 vs SCD MPs. (F) Effects of synthetic heme-laden MLVs preincubated with annexin-a5 or Hpx. *P < .05 vs none; #P < .05 vs SCD MPs.
Endothelial apoptosis by heme-laden MPs. Confluent HUVEC monolayers were treated with purified erythrocyte MPs (25 MPs/μL), synthetic heme-laden MLVs, or heme alone (5 μM) for 16 hours. Some MPs were preincubated with annexin-a5 (10 μg/mL) or Hpx (2 μM) for 1 hour. HUVECs were then fixed. Total DNA contents were determined by FACS after coloration with propidium iodide. Cells undergoing apoptosis (sub-G1 phase) or proliferation (G2/M) were quantified. (A) Representative DNA content profiles. We compared the effects of serial dilutions of control and SCD erythrocyte MPs (B) vs high serum (10% serum) or proapoptotic etoposide (100 μM). Triangles, SCD MPs; squares, control MPs. *P < .05 vs none; #P < .05 vs control MPs. (C) In other experiments, HUVECs were fixed in situ and stained with 4,6-diamidino-2-phenylindole. Nuclei displaying fragmentation, pyknosis, or condensed chromatin by fluorescence microscopy were counted as apoptotic and expressed as percentage. (D) Representative images (×400). Arrows designate fragmented and condensed nuclei. (E) Quantification of degraded nuclei after incubation with erythrocyte MPs (25 MPs/μL), vs high serum (10% serum), or proapoptotic etoposide. Some HUVECs were preincubated for 30 minutes with NAC (5 mM), DPI (10 μM), or apocynin (100 μM). *P < .05 vs none; $P < .05 vs control MPs; #P < .05 vs SCD MPs. (F) Effects of synthetic heme-laden MLVs preincubated with annexin-a5 or Hpx. *P < .05 vs none; #P < .05 vs SCD MPs.
Heme-laden MPs and mouse mesenteric vasodilation
We sought to establish the physiopathological significance of heme-laden MPs in vivo. We moved to the transgenic S-Antilles-D Punjab Hb-expressing (SAD) mouse model of SCD, a characterized model for vasoocclusions.14,20,41
First, we studied the plasma of SAD mice. We quantified circulating MPs by FACS and cell-free Hb by spectrophotometry (supplemental Figure 7A-B). SAD plasma contained approximately twice more circulating PS+ MPs and cell-free Hb than wild-type (WT) mice (n = 8, P = .035 vs WT). Plasma ultracentrifugation removed 70% to 90% of MPs (P < .05), as well as ∼30% of Hb in WT mice and more than 50% in SAD mice (P < .05 vs total). Concentrated stocks of heme-laden MPs were generated from SAD erythrocytes and purified.20
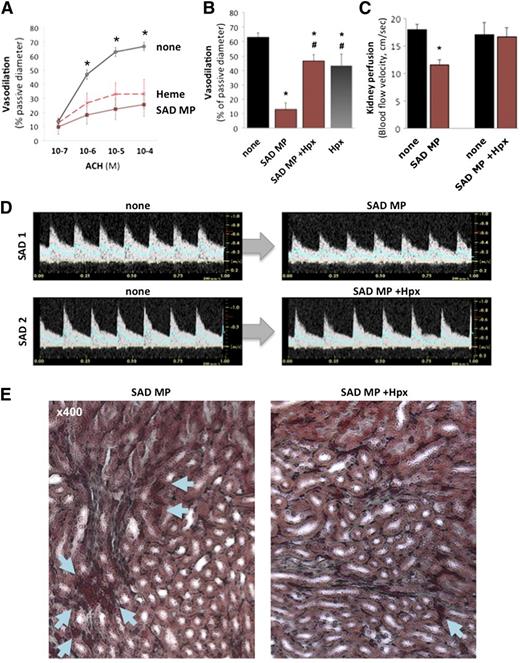
Next, we mounted mouse mesenteric resistance arterioles in pressure myographs to study endothelial function.20 PSS, heme (100 nM), or SAD MPs (300 MPs/μL) were perfused, and endothelium-dependent vasodilation was measured with ACH (Figure 5A). SAD MPs and heme strongly reduced ACH-dependent vasodilation (n = 12, P < .005 vs none). However, MPs incubated with Hpx (1 μM, 1 hour) lost their effect (Figure 5B; P < .01 vs MPs alone). Finally, a concentrated NO donor (sodium nitroprusside, 10−4 M) was superfused to determine maximal vasodilation (supplemental Figure 7C) and confirm the ability of vessels to dilate.
Erythrocyte MP heme induces endothelial damage and vasoocclusions. Mouse mesenteric resistances arteries were perfused with PSS alone, and initial diameters provided controls. Arterioles were then preconstricted with phenylephrine, and endothelium-dependent vasodilation was assessed in response to ACH (10−7 to 10−4 M). Arterioles were washed, constricted again with phenylephrine, and perfused with either SAD erythrocyte MPs (300 MPs/μL), or heme (100 nM) at 75 mm Hg pressure and 20 μL/s flow. (A) Endothelium-dependent vasodilation in response to increasing ACH doses (10−7 to 10−4 M) was quantified and expressed as percentage of passive diameter. *P < .05 vs SAD MPs (brown line) and heme (red dashes). (B) Some SAD MPs were pretreated with Hpx (1 µM, 1 hour) prior to perfusion. *P < .05 vs control; #P < .05 vs SAD MPs alone (+ none). To evaluate vasoocclusions in vivo, we injected 2 × 104 SAD erythrocyte MPs per mouse (brown) intravenously to SAD transgenic mice. We monitored kidney vasoocclusions by recording echo-Doppler velocity waveforms (C-D) and hemodynamic parameters. In each SAD mouse, we recorded the mean blood flow velocity (cm/s) in the right renal artery (blue line in Doppler velocity waveforms), before (none) or after intravenous injection of 2 × 104 SAD mouse erythrocyte MPs. Some MPs were preincubated with Hpx as previously described prior to injection. *P < .05 vs none. (E) Histologic analysis by Masson trichrome staining of SAD kidneys, 5 minutes after injection of SAD MPs, alone or preincubated with Hpx. Arrows show erythrocytes, with larger deposits and vascular congestion in SAD.
Erythrocyte MP heme induces endothelial damage and vasoocclusions. Mouse mesenteric resistances arteries were perfused with PSS alone, and initial diameters provided controls. Arterioles were then preconstricted with phenylephrine, and endothelium-dependent vasodilation was assessed in response to ACH (10−7 to 10−4 M). Arterioles were washed, constricted again with phenylephrine, and perfused with either SAD erythrocyte MPs (300 MPs/μL), or heme (100 nM) at 75 mm Hg pressure and 20 μL/s flow. (A) Endothelium-dependent vasodilation in response to increasing ACH doses (10−7 to 10−4 M) was quantified and expressed as percentage of passive diameter. *P < .05 vs SAD MPs (brown line) and heme (red dashes). (B) Some SAD MPs were pretreated with Hpx (1 µM, 1 hour) prior to perfusion. *P < .05 vs control; #P < .05 vs SAD MPs alone (+ none). To evaluate vasoocclusions in vivo, we injected 2 × 104 SAD erythrocyte MPs per mouse (brown) intravenously to SAD transgenic mice. We monitored kidney vasoocclusions by recording echo-Doppler velocity waveforms (C-D) and hemodynamic parameters. In each SAD mouse, we recorded the mean blood flow velocity (cm/s) in the right renal artery (blue line in Doppler velocity waveforms), before (none) or after intravenous injection of 2 × 104 SAD mouse erythrocyte MPs. Some MPs were preincubated with Hpx as previously described prior to injection. *P < .05 vs none. (E) Histologic analysis by Masson trichrome staining of SAD kidneys, 5 minutes after injection of SAD MPs, alone or preincubated with Hpx. Arrows show erythrocytes, with larger deposits and vascular congestion in SAD.
Heme-laden MPs and mouse kidney vasoocclusions
We proceeded to characterize the impact of heme-laden MPs on kidney vasoocclusions in mice and their association with kidney injury in patients.
In SAD mice, intravenous administration of SAD MPs induced a sudden 25% increase in circulating erythrocyte MPs.20 This reduced diastolic and mean blood flow velocity by 30% in renal arteries, within 5 minutes (Figure 5C-D; n = 6, P = .009 vs none). Histologic analysis of kidneys by Masson trichrome revealed erythrocyte packing and vascular congestion in the medulla (Figure 5E). MPs did not affect cardiac output or heart rate (supplemental Figure 7D-E). Conversely, SAD MPs preincubated with Hpx (1 μM) failed to modify blood flow velocity (P = .72 vs MPs alone) or histology.
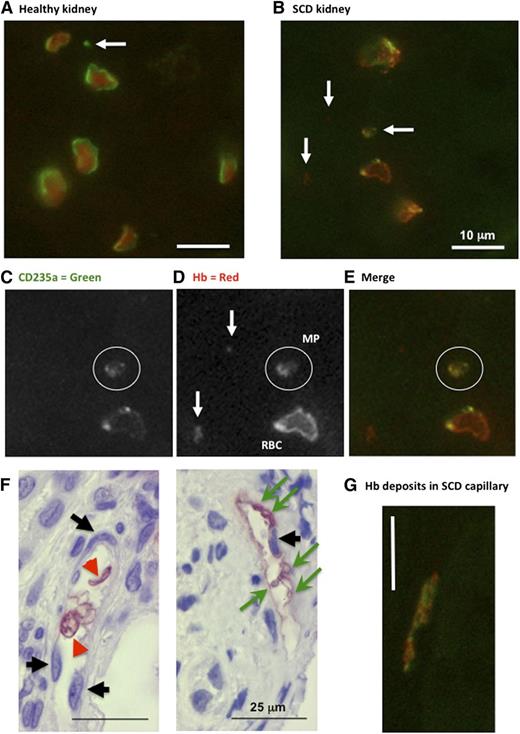
Next, we studied the distribution of Hb and CD235a (glycophorin-A) in human renal biopsies and peripheral tumorectomy samples by fluorescence and enzymatic immunohistochemistry. In healthy peritumoral kidney, erythrocytes presented circular shapes, homogenous Hb-filled cytoplasm, and strong CD235a+ cell-surfaces (Figure 6A). SCD erythrocytes presented distorted shapes with Hb-filled cytoplasm, but also submembrane concentrated Hb deposits and spotty CD235a+ labeling (Figure 6B-E,G). It was possible to identify CD235a+, Hb+ or CD235a+/Hb+ material compatible with larger MP sizes. We also noted that Hb deposits at the surface of nucleated vascular wall cells in capillaries in absence of nearby erythrocytes, was sometimes associated to circular vesicles compatible with larger MPs. Healthy capillary endothelium remained completely negative.
Immunohistochemistry for CD235a (glycophoryin) and Hb in human renal biopsies. Fluorescence immunohistochemistry in human normal peritumoral control (A) and SCD (B-G) kidney sections (n = 2) reacted with anti-human Hb and anti-human CD-235a IgG. High magnification micrographs were taken from tubulo-interstitial area (×600; white bars = 10 μm). White arrows identify CD235a+ or Hb+ particles. White circles surround a double-stained MPs. Note the submembrane deposition of Hb within SCD erythrocytes (C-E). (F) Immunohistochemistry with anti-human Hb IgG (×400). Black arrows identify counterstained capillary endothelial cell nuclei. Green arrows show Hb-positive cell fragments, and red arrowheads point at intact erythrocytes. Red stain reveals Hb in intact erythrocytes or deposited at the surface of nucleated vascular wall cells. (G) Fluorescence immunohistochemistry (×600) showing vascular Hb deposits.
Immunohistochemistry for CD235a (glycophoryin) and Hb in human renal biopsies. Fluorescence immunohistochemistry in human normal peritumoral control (A) and SCD (B-G) kidney sections (n = 2) reacted with anti-human Hb and anti-human CD-235a IgG. High magnification micrographs were taken from tubulo-interstitial area (×600; white bars = 10 μm). White arrows identify CD235a+ or Hb+ particles. White circles surround a double-stained MPs. Note the submembrane deposition of Hb within SCD erythrocytes (C-E). (F) Immunohistochemistry with anti-human Hb IgG (×400). Black arrows identify counterstained capillary endothelial cell nuclei. Green arrows show Hb-positive cell fragments, and red arrowheads point at intact erythrocytes. Red stain reveals Hb in intact erythrocytes or deposited at the surface of nucleated vascular wall cells. (G) Fluorescence immunohistochemistry (×600) showing vascular Hb deposits.
Counterstained enzymatic immunohistochemistry confirmed the localization of Hb deposits with respect to vascular cells (Figure 6F). We distinguished capillary endothelial cells in tubulo-interstitial (supplemental Figure 8A-D) and intraglomerular regions (supplemental Figure 8E-F). Healthy vascular walls remained devoid of Hb (supplemental Figure 8A,C,E). Intraglomerular and tubulo-interstitial capillary endothelium in dysfunctional kidneys of albuminuric SCD patients with glomerulopathy displayed conspicuous Hb staining, even away from erythrocytes (supplemental Figure 8B,D,F). Hb deposition on vessels was often associated to circular membrane structures with a diameter of ∼1:20th to 1:10th of nearby erythrocytes, compatible with Hb-laden MPs. Some Hb deposits clearly stained for CD235a, suggesting colocalization with erythrocyte membrane fragments (Figure 6G). The distribution of CD235a and Hb was however garbled in these deposits.
Discussion
Cell-free heme is associated with circulating MPs in SCD
Consistent with previous reports, we observe increased cell-free heme and erythrocyte MPs in plasma of SCD patients. Depletion of 80% of MPs removed ∼35% of heme in SCD plasma and <10% in controls. Membrane patterns, sizes and association with heme were similar in MPs prepared from purified human SCD and control erythrocytes. This is the first demonstration that a proportion of cell-free Hb and heme may not remain membrane-free in human plasma, but bind to cell membrane fragments of a diameter, density and composition compatible with MPs.
Met-Hb represented ∼20% of Hb species in plasma MPs, and the majority (>70%) of Hb associated with erythrocyte MPs generated in vitro. Met-Hb is one of the Hb species most liable to release hydrophobic heme that exerts oxidative damage to membranes and proteins. When incubated with endothelial cells in vitro or infused into SCD mice, heme-laden MPs caused strong and acute endothelial toxicity. Intravascular hemolysis and erythrocyte vesiculation may thus combine to produce heme-laden MPs and mediate endothelial injury. RBC MPs contain heme and Hb even when generated in the absence of extracellular heme or Hb. MP heme may thus largely derive from RBC, either by accumulation in membranes prior to, or by transfer of cytoplasm during, vesiculation. Experiments with synthetic phospholipid vesicles suggested that MPs from nonerythrocyte Hb-free cells may also capture free heme and disseminate it in the vasculature.
Extracellular Hb and heme may also bind to haptoglobin and Hpx, or to lipid structures other than MPs in SCD plasma.43 Lipoproteins are much smaller entities than MPs and require higher centrifugation speeds and times to sediment. Electron microscopy of SCD plasma pellets confirmed the presence of MPs but not lipoprotein complexes. SCD patients display moderate hypocholesterolemia, with reduced high-density lipoprotein cholesterol and apolipoprotein A-I.44,45 Our present work thus suggests that RBC MPs carry heme in SCD plasma. Other types of tissue injury, such as bacterial infections or ischemia, might also stimulate erythrocytes, promote vesiculation and shift heme compartmentalization toward MPs.
Heme-membrane interactions
Hb-S has been shown to interact with PS at physiological salt and pH.46 Our data show that met-Hb is associated with both plasma MPs and purified erythrocyte MPs, suggesting that cell-free Hb in plasma is oxidized to met-Hb. Met-Hb releases heme readily from its prosthetic pocket and this is enhanced by repeated cycles of deoxygenation and polymerization.3,47 Heme, released from Hb, is lipophilic and highly membranotropic.18,48,49 This pathway may explain why SCD erythrocytes contain excessive globin-free heme within their plasma membrane. Macroscopic clusters of hemichrome, known as Heinz bodies, with denatured Hb and heme closely associated with the inner leaflet of the plasma membrane.50 Hb accumulation on the cytoplasmic face of erythrocyte membranes could indeed be detected by immunohistochemistry in human SCD kidneys (Figure 6G). We speculate that Hb and heme deposits might be shed with MP during vesiculation and intravascular hemolysis.
We also observe that synthetic phospholipid vesicles avidly bind heme. Previous studies of heme-phospholipid interactions suggested a role for hydrophobicity, charge and pH.48,49 Our dynamic molecular simulations predicted that electrostatic heme:PS interactions might also exist on phospholipid surfaces, coordinated by Ca2+ ions. PS may not be necessary for heme movement toward hydrophobic pockets in phospholipid bilayers, but PS-rich domains were predicted to help retain some heme at the surface. This is compatible with the co-localization of heme deposits and PS-rich domains.43,50-53 In contrast, HbAA may develop weaker membrane interactions, and remain largely hydrosoluble in the MP cytoplasm.
The possibility remains that circulating phospholipid vesicles, even those from cells that do not normally contain Hb, scavenge cell-free heme. This would increase the probability that plasma heme is also captured by circulating MPs, regardless of cell origin. Conversely, the interaction of heme with membrane phospholipids and MPs may facilitate its scavenging by Hpx. Incubating SCD RBC MPs with Hpx, or scavenging Ca2+ with EDTA/EGTA significantly reduced their heme charge. This supports the idea that some heme can be retained at the surface of MP membranes, partly through divalent cation-mediated interactions. MP heme might then target cell surface receptors, such as TLR-4 and trigger signaling pathways similar, in part, to those described for soluble heme, including ROS production.25,26
MPs transfer heme to endothelial cell membranes and trigger oxidative stress
Erythrocyte MPs and synthetic heme/Hb-laden MLVs adhered to cultured endothelium and mediated a rapid accumulation of heme and Hb on endothelial surfaces in an Annexin-a5-sensitive fashion. Externalized PS and membrane contacts may thus contribute to the accumulation of heme on target vascular cells. Hb accumulated on the luminal surface of endothelium in human SCD renal biopsies, particularly on tubulo-interstitial or intraglomerular capillaries. These vessels are putative sites of intravascular hemolysis, with small internal diameter and strong biomechanical constraints for erythrocytes, and their function is deteriorated in SCD.
Once interacting with phospholipids, heme is thought to become highly prooxidant, either directly or after releasing its iron atom.8 H2O2 facilitates the opening of the heme ring and the release of iron. This free iron can move to lipophilic spaces of the plasma membrane, where it transfers electrons to nearby moieties, producing severe oxidative stress.
In culture, heme-laden erythrocyte MPs and MLVs, but not naked vesicles, triggered significant endothelial ROS production and eventual apoptosis. Conversely, apocynin and NAC blocked both ROS production and endothelial apoptosis. This suggested that heme-laden MPs are cytotoxic through oxidant activity, a conclusion compatible with our previous observation that ROS inhibitors block the effects of erythrocyte MPs on endothelial-dependent microvascular dilation ex vivo.20
Heme-laden MPs: an opening for novel therapeutic approaches
Using the SAD mouse model,14,41 we previously demonstrated that erythrocyte MPs can trigger rapid vasoocclusions in tissues that are sensitive to ischemic injury, including kidneys.20 Our current observations suggest that heme-laden MPs may have a significant pathophysiological impact in hemolytic disorders like SCD via reduced vasodilation, rapid vasoocclusions, and a possible later effect on vascular integrity. Moreover, our experimental conditions suggest that the impact of MPs on vascular function may be mediated by endothelial injury rather than NO chelation by Hb, an unlikely phenomenon in our experimental conditions.
In control plasma, Hpx is known to scavenge cell-free heme and provide systemic protection against oxidative stress. In vitro, Hpx could capture heme and separate part of it from SCD erythrocyte MPs, abrogating subsequent endothelial ROS production and apoptosis. In vivo, Hpx also prevented heme-laden MPs from compromising microvascular dilation, or inducing vasoocclusions. This implies that MP-associated heme, probably at the MP surface, is a primary effector of endothelial toxicity. Conversely, circulating Hpx may return some MP-bound heme to a non-MP fraction of plasma.
In summary, erythrocyte MPs may represent novel vectors of VOC and cardiovascular injury in SCD, because of their enrichment in heme and their increased levels during hemolysis. This may open novel therapeutic opportunities to manage plasma heme by Hpx supplementation or annexin-a5 administration.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Chantal Mandet of Hôpital Européen Georges Pompidou, Assistance Publique-Hôpitaux de Paris (Paris, France) for expert technical assistance with immunohistochemistry; Dr Alain Brisson of Bordeaux University (Talence, France) for providing MLV and LUV vesicles; Dr Slim Azouzi of the Institut National de Transfusion Sanguine (Paris, France) for expert advice on heme biology; Ghislaine Frébourg from Electron Microscopy Facility (FRE 3595, CNRS-Université Pierre et Marie Curie) for cryo-electron microscopy; Dr Eric Thervet from Nephrology Ward of Hôpital Européen Georges Pompidou for providing kidney biopsies; and Dr Eric Camerer for editing language.
This work was supported by the Agence Nationale pour la Recherche (ANR-Physio2007 Vasc-TSP1 [O.P.B.-B. and L.L.], ANR-MRare2008 SCAdhesion [P.-L.T.], ANR-11-IDEX-05-02 Recherche-USPC “HEMIR” [O.P.B.-B. and S.L.J.]), CNRS, and INSERM. S.M.C. benefited from a French government Ministère de l′Education Nationale de la Recherche et de la Technologie doctoral fellowship and a grant from Fondation pour la Recherche Médicale (FDT 2011-0922471). J.A.D.M. was funded by the Franco-Brazilian exchange program CAPES-COFECUB (629/09) (O.P.B.-B. and C.B.-F.).
Authorship
Contribution: O.P.B.-B., S.M.C., P.-L.T., and C.M.B. performed conception and design; O.P.B.-B., P.-L.T., L.L., C.B.-F., A.A., C.M.B., and A.T. obtained financial support; S.M.C., J.A.D.M., P. Bonnin, P.A., L.G., L.L., J.-C.L., D.C., J.-M.R., L.K., P. Bruneval, and O.P.B.-B. collected and/or assembled data; S.L.J., F.L., C.L., and H.L.C. performed patient recruitment; O.P.B.-B., S.M.C., J.A.D.M., P. Bonnin, P. Bruneval, and L.L. performed data analysis and interpretation; S.M.C. and O.P.B.-B. composed the manuscript; and O.P.B.-B. gave final approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olivier Blanc-Brude, Paris Center for Cardiovascular Research, Inserm U970, Hôpital Européen Georges Pompidou, 56 rue Leblanc, F-75015 Paris, France; e-mail: olivier.blanc-brude@inserm.fr.