Key Points
Using imatinib to treat CML first-line, with selective nilotinib switching, leads to excellent molecular response and survival.
This strategy may be preferable to universal first-line use of more potent agents, considering efficacy, toxicity, and economic factors.
Abstract
The Therapeutic Intensification in De Novo Leukaemia (TIDEL)-II study enrolled 210 patients with chronic phase chronic myeloid leukemia (CML) in two equal, sequential cohorts. All started treatment with imatinib 600 mg/day. Imatinib plasma trough level was performed at day 22 and if <1000 ng/mL, imatinib 800 mg/day was given. Patients were then assessed against molecular targets: BCR-ABL1 ≤10%, ≤1%, and ≤0.1% at 3, 6, and 12 months, respectively. Cohort 1 patients failing any target escalated to imatinib 800 mg/day, and subsequently switched to nilotinib 400 mg twice daily for failing the same target 3 months later. Cohort 2 patients failing any target switched to nilotinib directly, as did patients with intolerance or loss of response in either cohort. At 2 years, 55% of patients remained on imatinib, and 30% on nilotinib. Only 12% were >10% BCR-ABL1 at 3 months. Confirmed major molecular response was achieved in 64% at 12 months and 73% at 24 months. MR4.5 (BCR-ABL1 ≤0.0032%) at 24 months was 34%. Overall survival was 96% and transformation-free survival was 95% at 3 years. This trial supports the feasibility and efficacy of an imatinib-based approach with selective, early switching to nilotinib. This trial was registered at www.anzctr.org.au as #12607000325404.
Introduction
The International Randomized Study of Interferon Vs STI571 (IRIS) study reported 8-year overall survival (OS) of 85% in imatinib-treated chronic phase chronic myeloid leukemia (CP-CML) patients in the first-line setting.1 This is an excellent result, although not all patients benefited equally. In that cohort, 45% discontinued treatment with imatinib, mainly as a result of intolerance, drug resistance, or disease progression. The IRIS trial also demonstrated the important correlation between the achievement of time-dependent treatment targets and OS.2 Early cytogenetic and molecular response (MR) to treatment predicted subsequent disease response and event-free survival.3,4 Landmark analysis of the IRIS cohort demonstrated superior 5- and 7-year outcomes for patients achieving complete cytogenetic response (CCR) at 12 months and major molecular response (MMR) at 18 months compared with those who did not achieve those goals, a finding confirmed subsequently by other cohorts.2,5-10 On the basis of these results, together with similar findings from other studies, the European Leukemia Net (ELN) recommended in 2009 the achievement of partial cytogenetic response, CCR, and MMR by 6, 12, and 18 months of treatment, respectively, as being optimal responses for CP-CML patients.11 The prognostic significance of these targets was confirmed in patients treated with nilotinib12 and dasatinib13 in the first-line setting.
To improve outcomes in CP-CML patients, the Australasian Leukaemia and Lymphoma Group conducted the TIDEL-I trial, which used a higher imatinib starting dose of 600 mg/day for all patients and set a series of time-dependent treatment targets. These included complete hematologic response, major cytogenetic response, CCR, and BCR-ABL1 ≤0.01% (on the International scale [IS]) at 3, 6, 9, and 12 months, respectively. Patients failing to achieve these targets were treated with an increased imatinib dose of 800 mg/day. The cumulative incidence of MMR was 47% at 12 months and 73% at 24 months.14 By comparison, the 12-month MMR rate was 40% and 55% at these time points for the IRIS study.14
The TIDEL-II study aimed to optimize treatment outcomes by maximizing the number of patients reaching ELN treatment milestones. Building on our experience from TIDEL-I,14 in which treatment intensification was delivered on the basis of early treatment targets, we incorporated 2 additional approaches. Early reports suggested a correlation between minimum serum imatinib concentration achieved (>1000 ng/mL) and the likelihood of achieving CCR and/or MMR,15,16 leading us to escalate the imatinib dose to 800 mg/day in patients with trough serum concentration <1000 ng/mL. The second approach consisted of a prompt switch to the more potent tyrosine kinase inhibitor (TKI) nilotinib for either imatinib intolerance or a suboptimal response, as defined by molecular targets. The TIDEL-II targets were intended to be more stringent than the contemporaneous ELN milestones11 to allow adequate time for response improvement and avoid treatment failures. On the basis of efficacy and tolerability data from TIDEL-I, we continued to use imatinib at 600 mg/day as initial therapy.14
Patients and methods
Patient enrollment
TIDEL-II is a single-arm, prospective, open-label trial enrolling CP-CML patients older than age 16 years within 6 months of diagnosis. Exclusion criteria included known HIV infection, pregnancy, renal or hepatic dysfunction (creatinine and bilirubin ≥1.5 × upper limit of normal, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma glutamyl transferase ≥2.5 × upper limit of normal), past history of pancreatitis, or QTc >480 msec. Patients with myocardial infarction <12 months from CML diagnosis or those with other clinically significant uncontrolled heart disease (eg, unstable angina, congestive heart failure) were also excluded. Up to 6 months of prior treatment with hydroxyurea or anagrelide was permitted. The study was carried out with the approval of human research ethics committees and in accordance with the Declaration of Helsinki.
Treatment
The TIDEL-II treatment schema is illustrated in supplemental Figure 1 (available online at the Blood Web site). Two sequential cohorts of 105 patients each were enrolled. All patients started treatment with imatinib 600 mg/day. Imatinib plasma trough level was measured on day 22, 24 hours after a previous imatinib dose. A level of <1000 ng/mL determined whether a patient would be dose escalated to 800 mg/day or, if this dose could not be tolerated, the maximum tolerated dose was given. All patients then had to meet a series of specific time-dependent molecular targets, defined as BCR-ABL1 of ≤10%, ≤1%, and ≤0.1% (IS) at 3, 6, and 12 months, respectively. These targets have subsequently been adopted by the ELN as optimal treatment responses to first-line TKI treatment in 2013.17 Cohort 1 patients who failed to achieve their molecular targets were dose escalated to imatinib 800 mg/day; those who failed to achieve that target after a further 3 months were switched to nilotinib 400 mg twice per day. Patients who were unable to dose escalate imatinib after failing to achieve a time-dependent MR switched to nilotinib without further delay. Contemporaneously, the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study was published, which demonstrated a lack of benefit for using imatinib 800 mg/day rather than 400 mg/day for CP-CML in terms of MMR achievement, and that 800 mg/day was difficult to maintain.18 After enrolling 105 patients to cohort 1, a protocol amendment allowed subsequent TIDEL-II patients to switch directly to nilotinib if they failed to achieve TIDEL-II targets. Imatinib dose-escalation was retained only for patients with low imatinib trough levels in cohort 2. Patients from either cohort meeting the loss of response criteria (see “End points and statistical procedures”) were switched from imatinib to nilotinib 400 mg twice per day.
In either cohort, patients who experienced grade 3 to 4 or persistent grade 2 nonhematologic imatinib toxicity were subjected to review by the study management committee and switched to nilotinib if appropriate. Imatinib and nilotinib dose reductions were permitted for toxicity management.
Monitoring
Blood counts and biochemistry assessments were performed once per week for 4 weeks, at 3 months, and once per quarter thereafter. Bone marrow biopsies for morphologic and cytogenetic examination were performed locally at diagnosis, at 6 and 12 months after imatinib commencement, and in the event of treatment failure. BCR-ABL1 transcripts were measured monthly for the first 3 months and then every 3 months. Assays were performed centrally at an international reference laboratory (SA Pathology, Adelaide, Australia) by using methodology previously published.19,20 Testing for BCR-ABL1 kinase domain mutations21 was triggered when a more than twofold rise in transcript level was detected, when a patient met the loss of response criteria, or for failure to achieve TIDEL-II molecular targets.
End points and statistical procedures
The primary end point was confirmed MMR (BCR-ABL1 ≤0.1% IS) at 12 months (±4 weeks), documented by 2 BCR-ABL1 quantitative real-time polymerase chain reaction (qRT-PCR) results of ≤0.1% IS on 2 consecutive occasions 3 months apart. The date of achievement of MMR was then considered to be the date of the first of the 2 qRT-PCR results ≤0.1%. This end point was calculated on an intention-to-treat basis: patients with missing values and those who withdrew from study were scored as failures. The Clopper-Pearson exact method was used to calculate 95% confidence intervals (CIs). MRs were also calculated by using the cumulative incidence function with study discontinuation for any reason as a competing risk.22-24 Complete molecular response (CMR) was defined as 2 consecutive measurements of undetectable BCR-ABL1 within 3 months using qRT-PCR sensitivity of ≥4.5 log,25,26 also backdated to the first of the 2 measurements as for MMR. MR4 and MR4.5 were previously defined.27 A BCR-ABL1 result of ≤1% was considered a surrogate for CCR on the basis of a strong correlation between the two, as previously published.5,28 Patient numbers in the 2 cohorts were not powered to allow for any comparisons; therefore, this was not attempted.
A loss-of-response event is defined as any of the following: loss of confirmed complete hematological response or major cytogenetic response; cytogenetic clonal evolution; a confirmed more than fivefold increase in BCR-ABL1 level from nadir to a level >0.1% resulting in loss of MMR; a greater than twofold increase from nadir in BCR-ABL1 level to a level of >10%; detection of >50% mutant BCR-ABL1; or disease transformation to accelerated phase/blast crisis (AP/BC). The durations of OS and transformation-free survival (TFS) (survival without AP/BC) were estimated by using the Kaplan-Meier method. Transformation events include AP/BC observed for patients who discontinued study.
Results
Overall response
Between November 2007 and March 2011, 210 patients were enrolled from 27 centers in Australia and New Zealand. Baseline characteristics are summarized in Table 1.
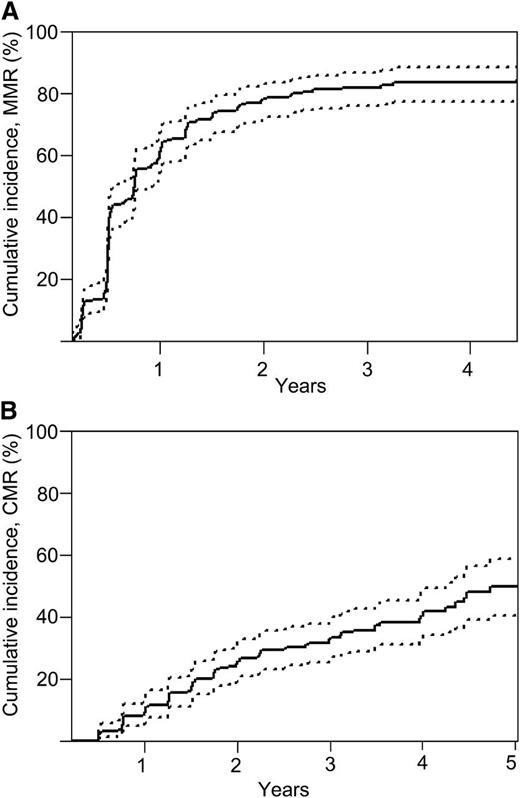
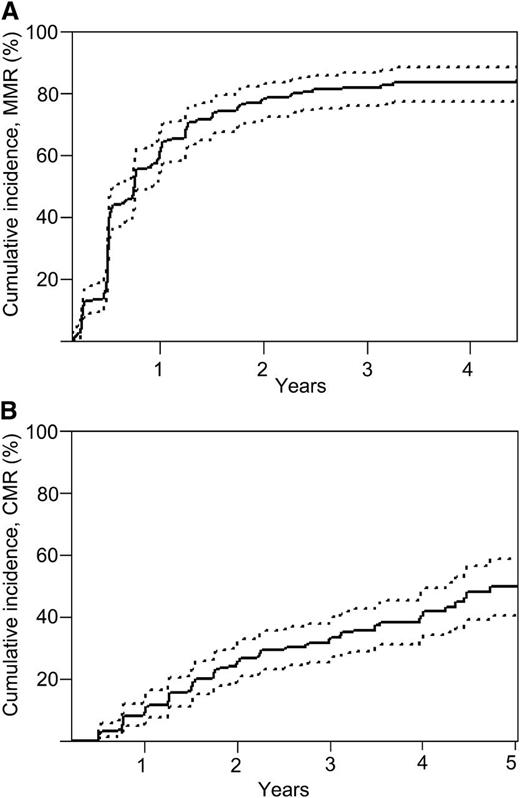
The number of patients achieving confirmed MMR and CMR in each of the cohorts is summarized in Table 2. Overall, 134 patients (64%; 95% CI, 56% to 72%) achieved confirmed MMR at 12 months, increasing to 153 patients (73%; 95% CI, 67% to 79%) at 24 months. The rates of MR4.5 were 19% at 12 months and 34% at 24 months. Confirmed CMR was achieved in 24 patients (11%; 95% CI, 6.8% to 15%) at 12 months and in 52 patients (25%; 95% CI, 19% to 31%) at 24 months. The cumulative incidence of achieving MMR and CMR are shown in Figure 1.
MRs. Cumulative incidence for (A) achievement of confirmed MMR (BCR-ABL1 ≤0.1% on 2 successive occasions) and (B) confirmed CMR. Point estimates are 60.9% (95% CI, 53.9% to 67.2%) at 12 months and 78.1% (95% CI, 71.8% to 83.1%) at 24 months for MMR. Point estimates are 9.0% (95% CI, 5.6% to 13.4%) at 12 and 25.2% (95% CI, 19.6% to 31.3%) at 24 months for CMR. Differences in visit window definition may contribute to the differences in the point estimates of confirmed MMR and CMR as measured by the cumulative incidence function noted in Table 2.
MRs. Cumulative incidence for (A) achievement of confirmed MMR (BCR-ABL1 ≤0.1% on 2 successive occasions) and (B) confirmed CMR. Point estimates are 60.9% (95% CI, 53.9% to 67.2%) at 12 months and 78.1% (95% CI, 71.8% to 83.1%) at 24 months for MMR. Point estimates are 9.0% (95% CI, 5.6% to 13.4%) at 12 and 25.2% (95% CI, 19.6% to 31.3%) at 24 months for CMR. Differences in visit window definition may contribute to the differences in the point estimates of confirmed MMR and CMR as measured by the cumulative incidence function noted in Table 2.
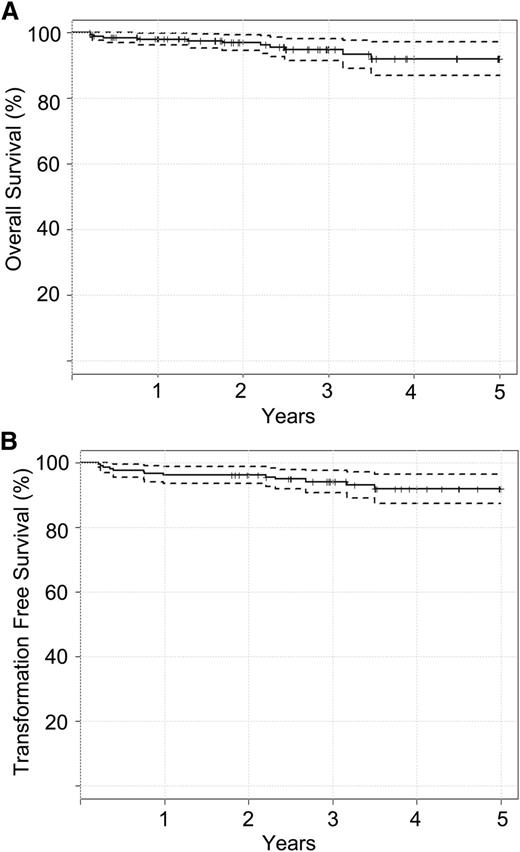
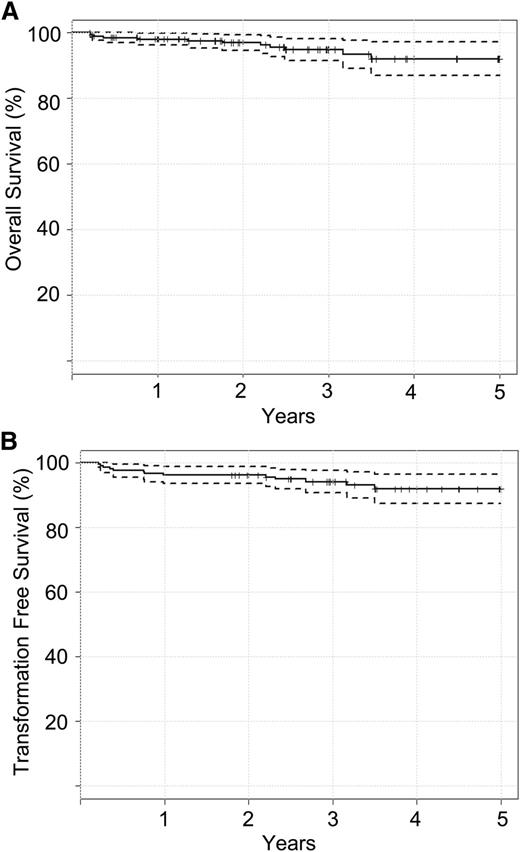
With a median follow up of 40 months, seven (3.5%) BC cases were observed. Six patients transformed while on study (at 3.5, 4, 5, 9, 12, and 34 months). One additional patient transformed to BC at 18 months after withdrawing from TIDEL-II at 12 months. No transformation to AP was observed. TFS was 95% (95% CI, 92% to 98%) at 3 years (Figure 2). Six additional patients died during the study as a result of causes not related to CML. OS was 96% (95% CI, 94% to 99%) at 3 years. Events such as transformation and death are summarized in supplemental Tables 1 and 2. Patients with high-risk Sokal scores were noted to have inferior OS, TFS, and achievement of MMR, although these differences did not reach statistical significance (supplemental Figure 2). Kinase domain mutations were detected in 11 patients (supplemental Table 3). Additional cytogenetic abnormalities at baseline were detected in only 8 patients and did not lead to statistically significant inferior outcomes (supplemental Table 4).
Survival curves calculated using the Kaplan-Meier estimate. (A) Overall survival and (B) transformation-free survival.
Survival curves calculated using the Kaplan-Meier estimate. (A) Overall survival and (B) transformation-free survival.
Treatment received by patients together with the discontinuation rate is summarized in Table 3 and supplemental Figure 3. Of the 134 patients in MMR at 12 months, 111 (83%) continued with imatinib therapy and 23 (17%) had been switched to nilotinib therapy. Of the 153 patients in MMR at 24 months, 111 (73%) were assigned to imatinib whereas 42 were assigned to nilotinib (27%). Of the 71 patients in MR4.5 at 24 months, 49 (69%) had only imatinib treatment.
Imatinib dose escalation for low trough levels at day 22
At day 22, 40 patients (19%) (19 from cohort 1 and 21 from cohort 2) recorded imatinib trough levels <1000 ng/mL (supplemental Figure 4). Dose was escalated for 31 of 40 patients at a median of 43 days (range, 31 to 187 days). Twenty patients remained on this dose until the 12-month time point, 2 de-escalated to imatinib 400 mg/day, 2 had withdrawn, and 7 switched to nilotinib (2 patients were intolerant and 5 failed to achieve the target). The other 9 patients had imatinib 400 to 600 mg/day as a maximum tolerated dose; 5 switched to nilotinib directly either for intolerance (n = 2) or for failing to reach their 3-month (n = 2) and 6-month targets (n = 1). One patient withdrew at 9 months, 1 later dose escalated at 13 months for failing to achieve MMR, and 2 achieved MMR on imatinib 600 mg/day. At 24 months, 28 (70%) of 40 achieved MMR; 15 of the 40 patients were still on imatinib 800 mg/day, 4 on imatinib 400 to 600 mg/day, 14 on nilotinib, and 7 had withdrawn from study.
EMR and outcome
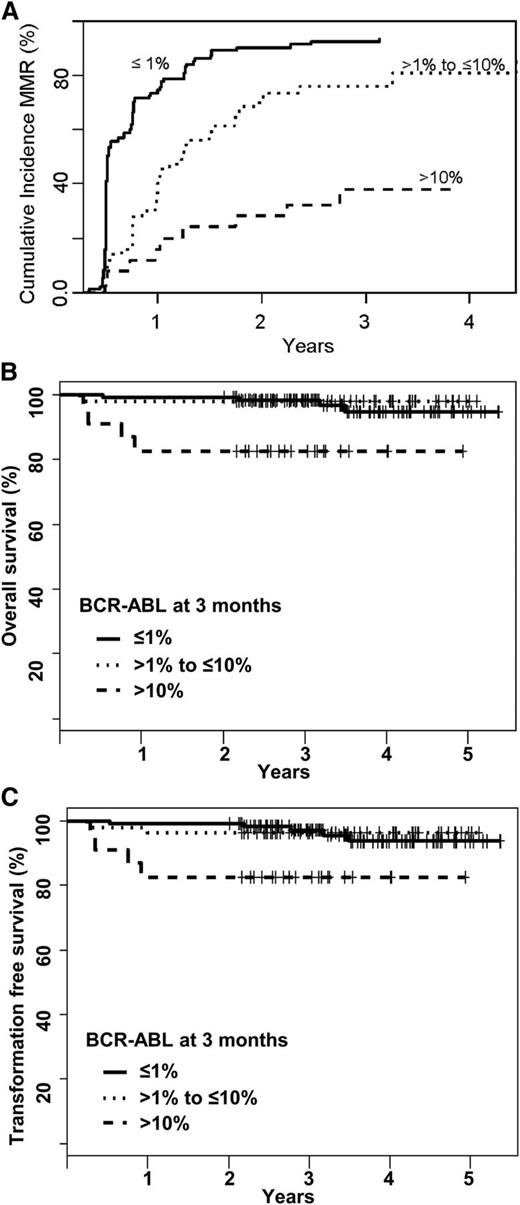
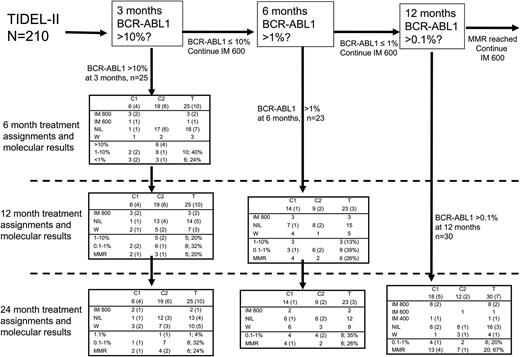
The 25 patients (12%) who failed to achieve an early molecular response (EMR; BCR-ABL1 ≤10% at 3 months) had inferior OS, TFS, and decreased probability of achieving MMR (Figure 3A-C). Six of these patients (24%) achieved MMR at 24 months. Ten of the 25 patients had imatinib plasma trough level <1000 ng/mL at day 22. At 6 months, 16 of these 25 patients had BCR-ABL1 <10% (6 patients, <1%), 6 had BCR-ABL1 >10%, and 3 had already withdrawn (1 from BC). At 12 months, 18 of 25 patients remained on study, 5 patients having achieved MMR. At 24 months, 6 (24%) of 25 patients were in MMR (2 on imatinib 800 mg/day and 4 on nilotinib), and 10 of 25 patients had withdrawn from the study: 3 each as a result of blastic transformation, failure to comply with protocol treatment, or failure to achieve satisfactory therapeutic responses (either MMR or CCR), and 1 patient died as a result of infection. In contrast, 91% of patients who achieved BCR-ABL1 ≤1% at 3 months subsequently achieved confirmed MMR at 24 months. In those with BCR-ABL1 between 1% and 10%, the 24-month rate of MMR was 75%. Treatment assignments for patients who failed to reach TIDEL-II time-dependent targets and subsequent molecular outcomes for other time points are described in Figure 4.
Outcomes stratified by a patient’s BCR-ABL1 level at 3 months in subgroups of >10%, ≤10% to >1%, and ≤1%. (A) Cumulative incidence for achievement of MMR, excluding 29 patients who achieved MMR prior to day 100. The differences among the 3 groups are statistically significant; P < .001 for each pairwise comparison. (B) OS by Kaplan-Meier method and (C) TFS. The survival differences among the 3 groups did not reach statistical significance for the survival analyses.
Outcomes stratified by a patient’s BCR-ABL1 level at 3 months in subgroups of >10%, ≤10% to >1%, and ≤1%. (A) Cumulative incidence for achievement of MMR, excluding 29 patients who achieved MMR prior to day 100. The differences among the 3 groups are statistically significant; P < .001 for each pairwise comparison. (B) OS by Kaplan-Meier method and (C) TFS. The survival differences among the 3 groups did not reach statistical significance for the survival analyses.
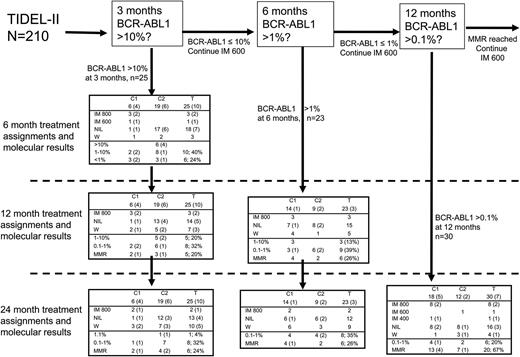
Treatment assignments and molecular outcomes for patients who failed to achieve TIDEL-II treatment targets. The proportion of patients with an imatinib trough level of <1000 ng/mL at day 22 is denoted by numbers in parentheses. Outcomes for the 25 patients who failed to achieve their 3-month molecular target of BCR-ABL1 ≤10% have been described in the article. Excluding the 25 patients who failed to achieve their 3-month target, 23 of the remaining 185 patients (11%) failed to achieve BCR-ABL1 <1% at 6 months. Fifteen switched to nilotinib by 12 months either directly or after a trial of imatinib 800 mg/day; another 3 remained on imatinib 800 mg/day, and 5 withdrew. At 24 months, 9 of the 23 had withdrawn (3 each as a result of toxicity, nonadherence to treatment protocol, or treatment failure). Of the remaining 14 patients, 2 remained on imatinib 800 mg/day and 12 remained on nilotinib; all had BCR-ABL1 <1%. Overall, 6 (26%) of the 23 patients had MMR at 24 months, only one doing so on escalated-dose imatinib. The 24-month MMR rate in this group is similar to that of patients with BCR-ABL1 >10% at 3 months. Of the 159 patients who achieved their 3- and 6-month TIDEL-II targets and had not withdrawn from the study before 12 months, 30 had BCR-ABL1 ≥0.1% at 12 months. At 24 months, 20 (67%) of these 30 patients had achieved and maintained MMR (10 on nilotinib, 9 on imatinib 800 mg/day, and 1 on imatinib 600 mg/day). Of the remainder, 6 had achieved and maintained BCR-ABL1 ≤1%; the other 4 of 30 withdrew from the study (2 with nilotinib-resistant mutations). Forty patients had trough imatinib levels <1000 ng/mL at day 22, 20 of whom subsequently failed to achieve 1 of the TIDEL-II molecular targets and are included in this diagram. This group of patients makes up 40%, 13%, and 23% of patients who failed to achieve their 3-, 6-, and 12-month targets, respectively. The other 20 patients all achieved MMR at 12 months, 15 of them having done so on imatinib 800 mg/day, 2 on imatinib 600 mg/day, and 3 on nilotinib (supplemental Figure 4). C1, cohort 1; C2, cohort 2; IM 400, imatinib 400 mg/day; IM 600, imatinib 600 mg/day; IM 800, imatinib 800 mg/day; NIL, nilotinib; T, total; W, withdrawn from TIDEL-II (MRs not recorded).
Treatment assignments and molecular outcomes for patients who failed to achieve TIDEL-II treatment targets. The proportion of patients with an imatinib trough level of <1000 ng/mL at day 22 is denoted by numbers in parentheses. Outcomes for the 25 patients who failed to achieve their 3-month molecular target of BCR-ABL1 ≤10% have been described in the article. Excluding the 25 patients who failed to achieve their 3-month target, 23 of the remaining 185 patients (11%) failed to achieve BCR-ABL1 <1% at 6 months. Fifteen switched to nilotinib by 12 months either directly or after a trial of imatinib 800 mg/day; another 3 remained on imatinib 800 mg/day, and 5 withdrew. At 24 months, 9 of the 23 had withdrawn (3 each as a result of toxicity, nonadherence to treatment protocol, or treatment failure). Of the remaining 14 patients, 2 remained on imatinib 800 mg/day and 12 remained on nilotinib; all had BCR-ABL1 <1%. Overall, 6 (26%) of the 23 patients had MMR at 24 months, only one doing so on escalated-dose imatinib. The 24-month MMR rate in this group is similar to that of patients with BCR-ABL1 >10% at 3 months. Of the 159 patients who achieved their 3- and 6-month TIDEL-II targets and had not withdrawn from the study before 12 months, 30 had BCR-ABL1 ≥0.1% at 12 months. At 24 months, 20 (67%) of these 30 patients had achieved and maintained MMR (10 on nilotinib, 9 on imatinib 800 mg/day, and 1 on imatinib 600 mg/day). Of the remainder, 6 had achieved and maintained BCR-ABL1 ≤1%; the other 4 of 30 withdrew from the study (2 with nilotinib-resistant mutations). Forty patients had trough imatinib levels <1000 ng/mL at day 22, 20 of whom subsequently failed to achieve 1 of the TIDEL-II molecular targets and are included in this diagram. This group of patients makes up 40%, 13%, and 23% of patients who failed to achieve their 3-, 6-, and 12-month targets, respectively. The other 20 patients all achieved MMR at 12 months, 15 of them having done so on imatinib 800 mg/day, 2 on imatinib 600 mg/day, and 3 on nilotinib (supplemental Figure 4). C1, cohort 1; C2, cohort 2; IM 400, imatinib 400 mg/day; IM 600, imatinib 600 mg/day; IM 800, imatinib 800 mg/day; NIL, nilotinib; T, total; W, withdrawn from TIDEL-II (MRs not recorded).
Outcomes of patients switching from imatinib to nilotinib
Seventy-eight patients failed to reach TIDEL-II targets. Fourteen patients remained on imatinib therapy (13 on 800 mg/day), and 12 of them achieved MMR at 24 months. Ten withdrew from study without further intervention. Fifty-four subsequently switched to nilotinib at a median of 7 months (range, 2 to 19 months) after study commencement. Twenty-one of these patients (39%) were in MMR at 24 months. Nineteen additional patients switched to nilotinib secondary to imatinib toxicity without failing any targets, at a median of 3 months after study commencement (range, 2 to 16 months), 5 doing so before 3 months. Six patients had already achieved MMR at the time of the switch. At 24 months, 18 (95%) of these 19 patients were in MMR. Altogether, 73 patients switched to nilotinib either for intolerance or for failure to achieve TIDEL-II targets, 33 of whom achieved MMR at 24 months. An additional 5 patients switched to nilotinib after loss of response to imatinib between 8 and 23 months (4 lost MMR; 1 lost CCR); 4 subsequently re-established MMR by 24 months.
Adverse events
Grade 3 to 4 adverse events (AEs) are listed in supplemental Table 5. These have a spectrum similar to those reported in other studies that use imatinib and nilotinib treatment. AEs attributed to the 2 drugs in this study are not directly comparable, because nilotinib exposure occurs subsequent to treatment with imatinib and only in selected patients (37% at 24 months). Cytopenia, especially at the beginning of study treatment, was the most common severe AE associated with imatinib. Biochemical abnormalities and allergic skin reactions were common with both imatinib and nilotinib treatment, the former including elevated serum liver or pancreatic enzymes for both drugs, as well as hypophosphatemia for imatinib and hyperbilirubinemia for nilotinib. Interestingly, grade 3 to 4 lipase and amylase elevations were seen with imatinib as well as nilotinib. Arthralgia, gastrointestinal disturbances, and edema were also commonly reported with imatinib. Vascular disease (involving coronary, cerebral, and/or peripheral arteries) was reported in 13 patients (6.2%) resulting in 5 deaths (summarized in supplemental Table 6). Most of these patients had preexisting vascular disease or significant vascular risk factors.
Discussion
The optimal first-line treatment of CML-CP patients remains unclear. Imatinib has been the standard of care since its introduction, associated with high OS and low risk of toxicity associated with major morbidity.2 In contrast, second-generation TKIs such as nilotinib and dasatinib lead to faster and deeper MRs,29,30 lower risks of AP/BC transformation, and in the case of nilotinib, lower risk of acquired kinase domain mutations.31 Second-generation TKIs are often preferred in patients with high Sokal or Hasford risk scores, or when a high priority is placed on the rapid achievement of deep MRs.32 However, outstanding questions remain over the long-term safety profile of these drugs. Although severe AEs such as vascular disease33-35 or pulmonary toxicities36,37 occur uncommonly with second-generation TKIs, these AEs lead to significant morbidity when they do occur. These toxicities may, in part, contribute to the lack of significant differences in OS between patients treated with imatinib and those receiving either nilotinib or dasatinib, despite the clear differences in AP/BC transformation rates.29,30
TIDEL-II represents a strategy that incorporated both imatinib and, where needed, a second-generation TKI into a treatment schema for first-line treatment of CML-CP patients. Overall, TIDEL-II treatment led to MMR rates of 64% by 12 months, increasing to 73% by 24 months. The 3-year OS and TFS are 96% and 95%, respectively. These results compare very favorably with other current first-line TKI studies in CML-CP patients, including the first-line use of nilotinib or dasatinib.29,30
TIDEL-II is a novel treatment strategy in many respects. Patients started treatment with imatinib 600 mg/day and modulated this dose according to serum imatinib trough levels, achievement of molecular targets, and tolerability. The overall effect of our individualized approach to imatinib administration led to 111 (53%) of the total 210 patients achieving MMR at 24 months on imatinib, of the 153 patients who achieved this end point. Although most clinical studies select 400 mg/day as the imatinib dose, an optimal dose for imatinib has never been established. The French STI571 Prospective Randomized Trial demonstrated superior MMR achievement with imatinib 600 mg/day vs 400 mg/day, although in the randomized TOPS study, MMR at 12 months was no different between patients randomly assigned to imatinib 400 mg/day and those assigned to 800 mg/day.18,38 The German CML IV study also randomly assigned patients to these 2 imatinib doses; the study found that the higher dose was well tolerated with a tolerability-adapted approach and that superior achievement of MMR/MR4 was demonstrated at 24 months with imatinib 800 mg/day.39 The higher median dose density achieved in the German study may have contributed to the differences not seen in TOPS.
The effect of imatinib 600 mg/day as the starting dose can also be demonstrated in the rate of EMR (BCR-ABL1 ≤10% at 3 months). Failure to achieve EMR correlates with inferior progression-free survival, OS, and achievement of MMR, irrespective of the TKI used for first-line treatment.4,9,10,12-14,26,40 Patients starting treatment with either dasatinib or nilotinib were more likely to achieve EMR compared with those receiving the standard dose of imatinib. The imatinib starting dose of 600 mg/day and dose escalation for low imatinib trough levels have likely contributed to our low EMR failure rate of 12%, which compares favorably to that seen in the nilotinib 300 mg twice per day arm of the Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients (ENESTnd) study (9%)12 and dasatinib-treated patients in the Dasatinib Versus Imatinib Study In Treatment-Naive CML study (16%).13 In contrast, 33% to 36% of patients in the imatinib 400 mg/day arm in these 2 studies failed to achieve EMR. Higher starting doses of imatinib have also been associated with a reduced rate of EMR failure in the Southwest Oncology Group S0325 study.41
We postulated that imatinib 800 mg/day may be beneficial for selected patients, such as those who failed to achieve milestone responses, although in practice, only 9 of our 28 patients mandated for dose escalation on the basis of target failure alone stayed on this dose until 24 months. We also hypothesized that dose escalation may be relevant in patients with low imatinib trough levels on the basis of retrospective data showing a correlation between achievement of CCR and/or MMR and a serum trough imatinib level >1000 ng/mL.15 This link was corroborated by a subanalysis of the IRIS study,16 although the clinical utility of imatinib serum levels has never been validated prospectively. In the 40 TIDEL-II patients with imatinib trough <1000 ng/mL, only 15 achieved MMR on imatinib 800 mg/day at 24 months. Taken together, ∼11% of patients can maintain imatinib 800 mg/day and achieve MMR on this dose, suggesting that the importance of high-dose imatinib may be secondary to therapy switching based on intolerance or failure to reach molecular targets.
Second, TIDEL-II allowed patients to switch from imatinib to nilotinib, which enabled an additional 32 patients (15% overall) to achieve MMR. Patients were allowed to switch for 3 reasons: imatinib intolerance, failure to achieve time-dependent molecular targets, or loss of response. Although switching TKIs for these reasons is a standard practice outside of clinical studies, patients in studies focused on a single TKI who switch drugs are usually regarded as therapeutic failures and are withdrawn from the study, with their subsequent outcome not reported. Incorporating this intervention within the TIDEL-II schema allowed us to observe real-world treatment outcomes that incorporate transition to second-line therapy.
Overall, 30% of TIDEL-II patients switched to nilotinib. For patients with imatinib intolerance, the outcome was generally favorable. In contrast, the benefit of nilotinib switching in patients who failed to achieve early TIDEL-II targets is not clearly demonstrated in our cohorts. This is a likely consequence of our low EMR failure rate. In the 300 mg twice per day arm of the ENESTnd trial, continuing nilotinib treatment in the 9% of patients with EMR failure led to a 24-month MMR rate of 29%.12 These patients are likely to have a degree of intrinsic TKI resistance through poorly understood mechanisms. It is reasonable to speculate that the 12% of TIDEL-II patients who failed to achieve EMR may have a similar degree of treatment resistance to a second-generation TKI, and nilotinib switching in this group of patients resulting in a 24-month confirmed MMR rate of 24% is therefore unsurprising. Furthermore, early transformations in these patients with relative TKI resistance suggests that even interventions as early as 3 months may be too late to have a clinically meaningful impact on underlying disease biology. For our 7 BC cases, 3 occurred in the first 6 months, and only 1 had an opportunity to receive the more potent TKI. However, it is doubtful that first-line dasatinib or nilotinib therapy would have prevented many of these early transformations, given the similar rate of transformation for patients treated with nilotinib and dasatinib compared with patients in the TIDEL-II study.12,13
TIDEL-II patients who failed to achieve later time-dependent targets had better outcomes compared with those who failed to achieve EMR. Although patients with BCR-ABL1 >1% at 6 months also had a low probability of achieving MMR at 24 months, BCR-ABL1 <1% (CCR equivalent) is still achievable in this group by using our approach. In contrast, interventions were more successful for those patients who failed to achieve BCR-ABL1 ≤0.1% at 12 months: 67% of these patients achieved MMR at 24 months (12 months after intervention). Furthermore, the overall strategy of TIDEL-II led to an MR4.5 rate of 34% at 24 months, compared with 25% in the nilotinib 300 mg twice per day arm of the ENESTnd study.42 Of the 71 patients who achieved confirmed MR4.5, 49 received only imatinib (23% overall). Nilotinib switching allowed an additional 22 patients (10% overall) to achieve this end point. A deep MR is an increasingly valued treatment goal associated with improved survival43,44 and acting as a platform for cessation studies aimed at treatment-free remissions.45,46 Results of our 12-month intervention and our overall MR4.5 rates are consistent with the results of the Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Complete Molecular Response study,47 which demonstrated achievement of deeper MRs after switching therapy from imatinib to nilotinib in patients who had achieved CCR but not MR.4.5
For the vast majority of CML patients, the TIDEL-II approach leads to excellent outcomes. Future efforts should be directed at early identification of high-risk patients (especially those destined to experience early disease transformation) and the development of experimental strategies targeted at this population. Ideally, prognostic biomarkers of sufficient discriminatory ability should be implemented at diagnosis. Clinical scoring systems such as the Sokal index,48 Hasford,49 and European Treatment Outcome Study 50 scores continue to have relevance for CML patients and may be useful in combination with emerging biomarkers51-60 in the derivation of a new prognostic score. Better salvage therapies targeted at high-risk patients may improve outcomes in these patients and spare unnecessary additional toxicity for the 60% to 80% of patients who do well with current regimens.
In conclusion, TIDEL-II represents a novel and effective treatment option for the management of treatment-naïve CP-CML patients. Although imatinib is effective treatment for many patients, we recognized that some patients will need a more potent kinase inhibitor. In contrast, using second-generation TKIs universally as first-line therapy may lead to increased long-term toxicity. TIDEL-II allows a cohort of patients to begin treatment on imatinib, with the majority achieving EMR and MMR while remaining on a drug with long-term safety data. The strategy allows selection of patients who are less sensitive to or are intolerant to imatinib, and switching them to nilotinib in a time-dependent manner to minimize treatment failure. Given the probability that generic imatinib will be increasingly available in many countries over the next decade, the TIDEL-II strategy is particularly attractive when the increasing economic burden of CML therapy is considered.61 We note that molecular monitoring may not be available or commonly practiced in some countries, which may limit application of strategies such as those in TIDEL-II. However, successive technological improvements will improve access to these assays, making such risk-adapted sequential agent strategies feasible. We believe strategies such as those in TIDEL-II may be preferable to the universal use of second-generation TKIs as first-line treatment, and formal comparative studies between the strategies are warranted.
Presented in part at the 52nd annual meeting of the American Society of Hematology (ASH), Orlando, FL, December 4-7, 2010; 53rd annual meeting of the ASH, San Diego CA, December 10-13, 2011; and the 54th annual meeting of the ASH, Atlanta, GA, December 8-11, 2012. Also presented at the 16th Congress of the European Hematology Association (EHA), London, United Kingdom, June 9-12, 2011.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Stella Vlahos and Kate Dunster (Biostatistics and Clinical Trials Centre at the Peter MacCallum Cancer Centre), all of the hospital study coordinators, and Stephanie Arbon and Bronwyn Cambareri (South Australia Health and Medical Research Institute) for their help during the clinical study and the preparation of the manuscript. The authors acknowledge Dr Wendy Parker for critically reviewing the manuscript and are grateful to all of the CML patients who participated in the study.
This work was supported in part by grants from Novartis Pharmaceuticals Australia. This study was sponsored by the Australasian Leukaemia and Lymphoma Group. D.T.Y. is a PhD candidate at the University of Adelaide and this work is submitted in partial fulfillment of the requirement for the PhD. D.T.Y. receives PhD scholarship funding from the Leukaemia Foundation of Australia and the Royal Adelaide Hospital Research Foundation A. R. Clarkson Scholarship. D.T.Y. received a Clinical Research Training Institute training award from the American Society of Hematology. T.P.H. is a National Health and Medical Research Council Practitioner Fellow. Novartis has no role in gathering, analyzing, or interpreting the data.
A list of investigators and participating centers is provided in the supplemental Data.
Authorship
Contribution: D.T.Y. supervised conduct of the study, contributed patients, gathered and analyzed the data, created the figures, and wrote the manuscript; M.P.O. and A.K.M. supervised conduct of the study, contributed patients, and reviewed the manuscript; D.L.W. designed the study and reviewed the manuscript; S.B. designed the study, analyzed molecular data, and reviewed the manuscript J. Braley analyzed molecular data and reviewed the manuscript; M.K., J. Beresford., and A.H. gathered and analyzed the data and reviewed the manuscript; S.I., D.K.H., M.H., A.P.S., R.F., C.K.A., Y.L.K., J. Trotman, C.J.F., J. Taper, and D.M.R. contributed patients and reviewed the manuscript; C.T. supervised the conduct of the study and reviewed the manuscript; A.P.G. and T.P.H. designed the study, supervised conduct of the study, contributed patients, served on the study management committee, and reviewed the manuscript.
Conflict-of-interest disclosure: D.T.Y. received research funding from Novartis and Bristol-Myers Squibb (BMS) and received honoraria from and participated on advisory boards of Novartis and BMS. D.L.W. and D.K.H. received research funding from Ariad, CSL, Novartis, and BMS and received honoraria from and participated on advisory boards of Novartis and BMS. S.B. received research funding from Ariad, Novartis, Otsuka, and BMS and received honoraria from and participated on advisory boards of Ariad, Novartis, Qiagen, and BMS. A.P.S. acted as a consultant for Novartis, BMS, and Pfizer and received honoraria from Novartis and BMS. C.K.A., A.P.G., and A.K.M. participated on advisory boards of Novartis and BMS. D.R.M. received research funding from Novartis and received honoraria from and participated on advisory boards of Novartis and BMS. T.P.H. received research funding from Ariad, CSL, Novartis, and BMS and received honoraria from and participated on advisory boards of Ariad, Pfizer, Novartis, and BMS. The remaining authors declare no competing financial interests.
Correspondence: Timothy P. Hughes, Department of Haematology, SA Pathology, PO Box 14, Rundle Mall, SA 5000, Australia; e-mail: timothy.hughes@health.sa.gov.au.