Key Points
Early thrombolytic treatment with a bispecific inhibitor against TAFI and PAI-1 is effective without exogenous tPA.
Even at the highest dose tested, the bispecific inhibitor against TAFI and PAI-1 does not prolong bleeding time.
Abstract
Circulating thrombin-activatable fibrinolysis inhibitor (TAFI) and plasminogen activator inhibitor-1 (PAI-1) are causal factors for thrombolytic failure. Therefore, we evaluated an antibody-engineered bispecific inhibitor against TAFI and PAI-1 (heterodimer diabody, Db-TCK26D6x33H1F7) in several mouse models of thrombosis and stroke. Prophylactic administration of the diabody (0.8 mg/kg) in a thromboplastin-induced model of thromboembolism led to decreased lung fibrin deposition. In a model of cerebral ischemia and reperfusion, diabody administration (0.8 mg/kg, 1 hour postocclusion) led to a mitigated cerebral injury with a 2.3-fold reduced lesion and improved functional outcomes. In a mouse model of thrombin-induced middle cerebral artery occlusion, the efficacy of the diabody was compared to the standard thrombolytic treatment with recombinant tissue-type plasminogen activator (tPA). Early administration of diabody (0.8 mg/kg) caused a twofold decrease in brain lesion size, whereas that of tPA (10 mg/kg) had a much smaller effect. Delayed administration of diabody or tPA had no effect on lesion size, whereas the combined administration of diabody with tPA caused a 1.7-fold decrease in lesion size. In contrast to tPA, the diabody did not increase accumulative bleeding. In conclusion, administration of a bispecific inhibitor against TAFI and PAI-1 results in a prominent profibrinolytic effect in mice without increased bleeding.
Introduction
Plasminogen activators are the only thrombolytic agents approved to rapidly revascularize a thrombosed vessel. Reperfusion of the ischemia-affected organ leads to an improved outcome in patients when applied within the first hours after ischemic onset.1 Despite this evidence-based beneficial effect, current thrombolytic agents remain widely underutilized due to life-threatening side effects such as cerebral hemorrhages and possible neurotoxicity.2,3 For acute ischemic stroke, in particular, the only licensed treatment option consists of a high systemic dose of recombinant tissue-type plasminogen activator (tPA), which is actually given to <10% of the patients. Therefore, there is an unmet clinical need to explore novel therapeutic avenues to enhance fibrinolysis without plasminogen activator–associated adverse effects. Endogenous intravascular fibrinolysis is driven on the release of tPA from the endothelium, which in turn enzymatically activates plasminogen into the fibrin-degrading enzyme, plasmin.4 This activation step is attenuated by circulating thrombin-activatable fibrinolysis inhibitor (TAFI) and plasminogen activator inhibitor-1 (PAI-1). Activated TAFI (TAFIa; encoded by the CPB2 gene) eliminates C-terminal Lys exposed on partially degraded fibrin, which ultimately leads to a diminished efficiency and localization of plasminogen activation by tPA.5 PAI-1 belongs to the serine protease inhibitor (serpin) superfamily and is the primary inhibitor of tPA. The active form of PAI-1 can irreversibly neutralize the activity of its target serine protease by forming a 1:1 stoichiometric covalent complex, accompanied by deformation of the catalytic triad of the serine protease.6
Accordingly, elevated plasma levels of TAFI, PAI-1, or both inhibitors instigate an increased risk for thrombotic disorders (eg, venous thromboembolism,7 acute ischemic stroke,8 and acute myocardial infarction9,10 ) and cause thrombolytic failure.11,12 A considerable number of studies using animal thrombosis models have demonstrated a beneficial effect of TAFI and PAI-1 as drug targets,13 but no study has yet shown thrombolytic superiority over plasminogen activators.14,15 We postulate that single targeting of the antifibrinolytic system does not suffice for robust thrombolysis. Because TAFI and PAI-1 act in a complementary way at the fibrin surface,16 we hypothesized that dual TAFI/PAI-1 targeting would allow efficient thrombolysis at safer systemic drug levels.
Highly specific monoclonal antibodies (MA) against TAFI and PAI-1 are available and have been previously validated in vivo.17-19 MA-TCK26D6 modulates TAFI/TAFIa by impairing activation mediated by thrombin and plasmin; however, it does not block the stronger activator, thrombin/thrombomodulin.17 Interestingly this antibody also prevents the specific interaction of TAFIa on fibrin, but not on the other small proinflammatory substrates of TAFIa.20 MA-33H1F7 converts the active form of PAI-1 to a substrate for tPA, leading to the irreversible cleavage of PAI-1.21 TAFI and PAI-1 both act in the vicinity of a fibrin clot, making simultaneous targeting of the 2 antigens through 1 protein a feasible and potentially safer strategy. Previously, a corresponding small recombinantly engineered bispecific antibody, a diabody, was developed to target TAFI and PAI-1 simultaneously and was designated as Db-TCK26D6x33H1F7.22 In a thromboelastometric assay, we confirmed that simultaneous inhibition of TAFI and PAI-1 causes an enhancement of in vitro fibrinolysis in human and mouse blood.22
In the present study, Db-TCK26D6x33H1F7 was used to evaluate the concept of dual TAFI/PAI-1 inhibition in models of thromboembolism and stroke. The thromboprophylactic capacity of the diabody was evaluated in a well-established mouse model of venous thromboembolism.17,18,23,24 In addition, the effect of the diabody on brain ischemia/reperfusion injury was assessed in a mechanical transient middle cerebral artery occlusion (tMCAo) model in which brain lesion and neurologic/motor outcome were measured. Furthermore, the profibrinolytic capacity was evaluated in 2 stroke models in which in situ clots are induced in the vascular lumen of the middle cerebral artery (MCA) via a thrombin- and a ferric chloride (FeCl3)-induced MCA occlusion (MCAo) model. The goal was to compare the efficacy of the diabody vs the standard thrombolytic treatment to tPA after thrombus formation. Finally, safety regarding potential bleeding risks was evaluated.
Methods
A detailed description of the methods is provided in the supplemental Methods, available on the Blood Web site.
Animals
Thromboplastin-induced thromboembolism, pharmacokinetics, and tail bleeding assay were performed on female SWISS mice (18-22 g; Janvier Labs, Saint Berthevin, France). Mechanical tMCAo was performed on C57BL/6J mice (females, 16-20 g; males, 20-24 g; The Jackson Laboratory). Thrombotic MCAo was performed on male SWISS mice (30-35 g; Janvier Labs). Experiments were performed in accordance with local ethical laws and local ethics committees (see supplemental Methods for details) and the Council of the European Communities Directive of November 24, 1986 (86/609/EEC) guidelines for the care and use of laboratory animals. All experiments were performed following Animal Research: Reporting of in vivo Experiments guidelines (www.nc3rs.org.uk), including randomization of treatment, as well as surgery and analysis blind to the treatment.
Venous thromboembolism model
Thromboplastin-induced thromboembolism was performed as described previously,17 with slight modifications. Endotoxemia was induced in overnight-fasted nonanesthetized mice by intraperitoneal injection of lipopolysaccharide (0.5mg/kg) from Escherichia coli serotype 026:B6 (Sigma-Aldrich, St Louis, MO) 3 hours prior to the start of the experiment. Then, diabody (0.2 mg/kg or 0.8 mg/kg) or vehicle (isotonic saline) was administered via tail vein IV injection in endotoxemic mice. Five minutes posttreatment, thromboembolism was induced by IV injection of thromboplastin (corresponding to a dose of 2.5µg/kg tissue factor). Fibrin deposition in lungs was quantified 15 minutes after thromboplastin injection as described previously17 (supplemental Methods).
Mechanical tMCAo
Surgical procedures and treatments.
Mechanical tMCAo was performed as described previously, according to the monofilament method.25 The intraluminal suture was left in situ for 60 minutes. The animals were then reanesthetized, and the occluding monofilament was withdrawn to allow reperfusion. Immediately after reperfusion, diabody (0.8 mg/kg) or vehicle (phosphate-buffered saline) was administered by IV injection.
Neurologic tests.
Lesion quantification and cerebral fibrin(ogen).
At 24 hours postocclusion, mice were euthanized to quantify lesion volumes through staining with 2% 2,3,5-triphenyltetrazolium chloride. Cerebral fibrin(ogen) was determined as described in “Protein extraction and western blot analysis” in the supplemental Methods.
Thrombotic (thrombin-induced) MCAo
Surgical procedure and treatments.
Deeply anesthetized mice by inhalation of 5% isoflurane/oxygen mixture were placed on a stereotaxic device and were maintained under anesthesia by inhalation of 2% isoflurane/oxygen mixture during surgical procedures. Body temperature was maintained at 37°C with a thermostat-controlled heating pad throughout the procedure. Right MCA was exposed by craniectomy. In situ occlusion of the MCA was performed by microinjection of murine α-thrombin (1 IU; Kordia Life Sciences, Leiden, The Netherlands) into the MCA,28 and time of occlusion was determined by laser Doppler flowmetry. Diabody or vehicle (phosphate-buffered saline) was administered by IV injection via a tail vein catheter at early (20 minutes), intermediate (90 minutes), and late (240 minutes) time points postocclusion. Five minutes after diabody or vehicle administration, tPA (10 mg/kg) or saline was administered by IV injection (10% as bolus and 90% infused over 40 minutes).
MRI analysis.
Magnetic resonance imaging (MRI) was performed at 24 hours postocclusion on a PharmaScan 7 T/12 cm system using surface coils (Bruker Biospin; Wissembourg, France). Brain lesion volume was determined by T2-weighted MRI (multislice, multiecho) sequence: repetition time/echo time of 2500 milliseconds/51 milliseconds29 ); the angiographic scoring of the MCA (0 = occlusion, 1 = partial recanalization, and 2 = complete recanalization) was determined by magnetic resonance angiography (2-dimensional time-of-flight sequences30 ); and cerebral hemorrhages were detected by T2*-weighted MRI (fast low-angle shot sequence: repetition time/echo time of 350 milliseconds/6 milliseconds). Mice with a lesion volume <3 mm3 were excluded (no mice were excluded from thrombin-induced MCAo experiments).
Tail bleeding assay
Mouse tail vein bleeding times and hemoglobin loss were determined with a tail-clipping assay, as described previously31 (supplemental Methods).
Statistical analysis
All quantitative data are presented as mean and standard error of mean (SEM). Statistical analysis was performed with GraphPad Prism v.5 (GraphPad Software) (supplemental Methods). P values <.05 were considered significant.
Results
Characterization of the profibrinolytic effect of diabody in a model of venous thromboembolism
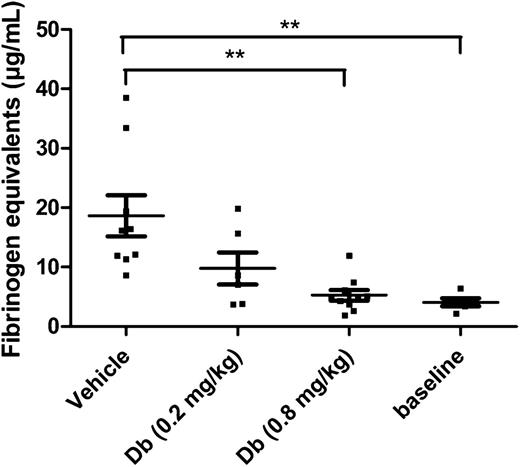
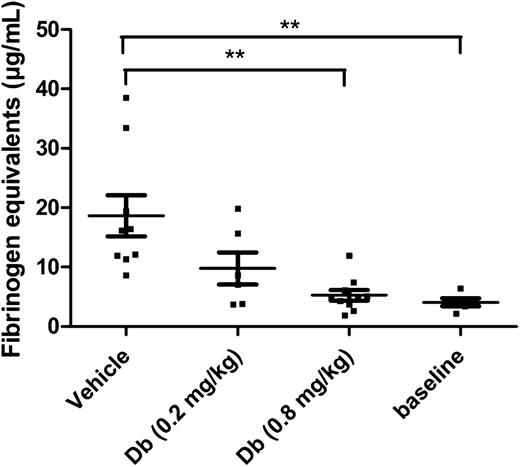
In a model of thromboplastin-induced thromboembolism, which is well established for the evaluation of profibrinolytic agents,17,18,23,32 the diabody was tested to determine an effective dose. Because of the extremely low baseline levels of PAI-1 in mice, endotoxemic mice were used in this type of acute model to obtain a potential contributing effect of PAI-1 inhibition. Previously it was reported that under the given conditions, endotoxemic mice had an increased PAI-1 plasma level of 112 ± 30 ng/mL.33 In the present study, the plasma level of TAFI was also measured under endotoxemic conditions and was found to be 6.5 ± 0.4 µg/mL (n = 3). An initial dose of 0.2 mg/kg was administered by IV injection to obtain a circulating concentration of diabody, theoretically equimolar to TAFI [TAFI plasma level (µg/mL) × plasma volume/body weight (mL/mg) × correction Mw: 6.5 µg/mL × 0.030 mL/mg × 58 kDa/56 kDa = 0.2 mg/kg]. At 0.2 mg/kg diabody, thromboplastin-induced fibrin deposition in lungs was reduced but not to a significant degree. With a higher dose of 0.8 mg/kg diabody, however, fibrin deposition in the lungs was maximally reduced (94% reduction; Figure 1; P < .01 vs vehicle, n = 10 mice/group). Five minutes after administration of 0.8 mg/kg diabody, the plasma level was 6.6 ± 0.2 µg/mL diabody (n = 3, supplemental Figure 2), which is consistent with the rapid initial clearance (t½α) of a diabody.34 Thus, administration of diabody at 0.8 mg/kg results in an approximately equimolar concentration to TAFI shortly after injection. The diabody was used at a dose of 0.8 mg/kg in further experiments, except for the thrombolysis experiments on FeCl3-induced thrombi (supplemental Figure 3), which are more resistant to fibrinolysis.
Thromboprophylactic effect of the diabody in a model of venous thromboembolism. Fibrin deposition in lungs from endotoxemic mice (n = 6-10), expressed in fibrinogen equivalents. Baseline levels were obtained by isolating lungs from healthy mice (n = 5) without thrombotic challenge. Data are represented as mean ± SEM. **P < .01. Db, diabody.
Thromboprophylactic effect of the diabody in a model of venous thromboembolism. Fibrin deposition in lungs from endotoxemic mice (n = 6-10), expressed in fibrinogen equivalents. Baseline levels were obtained by isolating lungs from healthy mice (n = 5) without thrombotic challenge. Data are represented as mean ± SEM. **P < .01. Db, diabody.
Profibrinolytic effect of diabody on cerebral ischemia/reperfusion injury
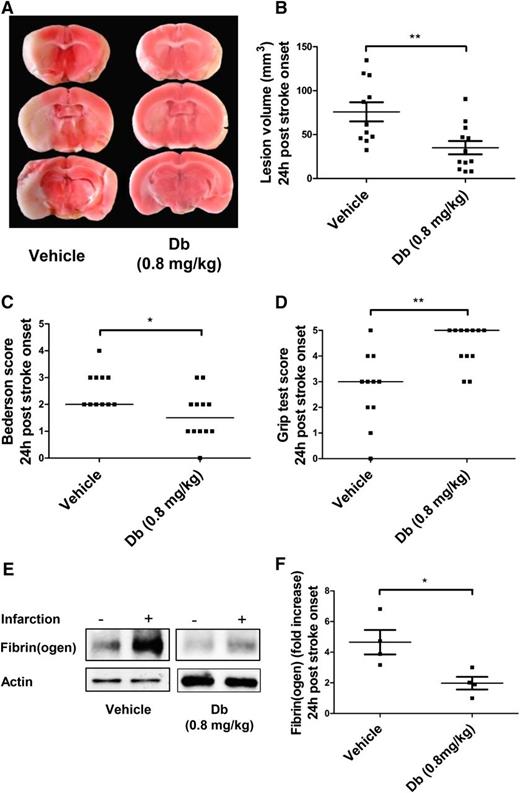
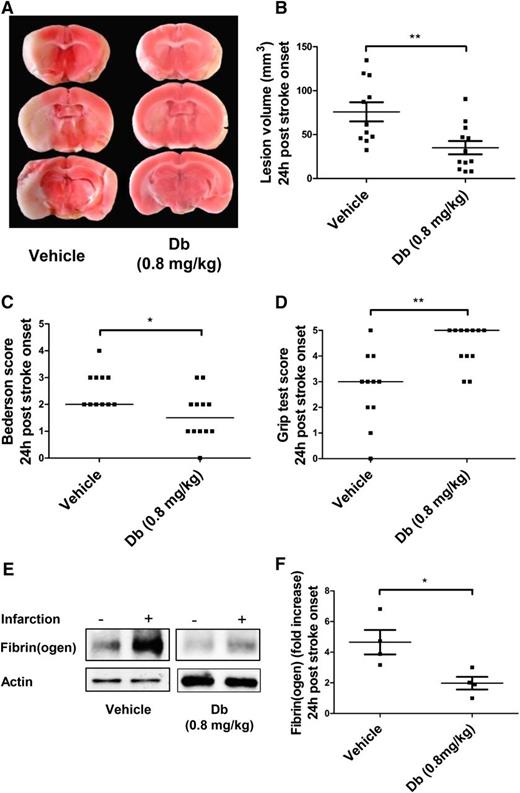
A mechanical tMCAo model was used to assess the effect of diabody on cerebral ischemia/reperfusion injury when administered at 1 hour postocclusion. This model typically yields large lesion volumes in untreated mice that have measurable neurologic/motor defects. Experiments on the pharmacokinetics of the diabody showed that its circulating half-life (t½β) is 121 minutes (supplemental Figure 2), which allows its administration by a single bolus. At 24 hours postocclusion, mice treated with diabody (0.8 mg/kg) exhibited significantly reduced lesion volumes (Figure 2A-B; P < .01; n = 10-12 mice per group) and concomitantly improved neurologic scores (Figure 2C; P < .05; n = 10-12 mice per group) and motor scores (Figure 2D; P < .01; n = 10-12 mice per group). In addition, western blot analysis revealed that the diabody effectively reduced massive fibrin deposition induced by reperfusion injury by at least twofold (Figure 2E-F; P < .05; n = 4 mice per group).
Evaluation of the diabody in a model of cerebral ischemia (60 minutes) and reperfusion. Representative triphenyltetrazolium chloride–stained brain slices (A), lesion volume (B), Bederson score (C), and Grip test score (D) of mice treated with vehicle or Db (0.8 mg/kg) alone on reperfusion (60 minutes postocclusion). (E) Representative immunoblots of ipsilateral vs contralateral hemispheres. (F) Fibrin(ogen) contents in ipsilateral hemisphere (fold increase vs contralateral hemisphere) of mice treated with vehicle or diabody (0.8 mg/kg) on reperfusion (60 minutes postocclusion). All parameters were measured 24 hours after stroke onset. Data are represented as mean ± SEM (panels B,F) or median (panels C-D); n = 10-12 mice per group (panels B-D); n = 4 mice per group (panel F). *P < .05; **P < .01.
Evaluation of the diabody in a model of cerebral ischemia (60 minutes) and reperfusion. Representative triphenyltetrazolium chloride–stained brain slices (A), lesion volume (B), Bederson score (C), and Grip test score (D) of mice treated with vehicle or Db (0.8 mg/kg) alone on reperfusion (60 minutes postocclusion). (E) Representative immunoblots of ipsilateral vs contralateral hemispheres. (F) Fibrin(ogen) contents in ipsilateral hemisphere (fold increase vs contralateral hemisphere) of mice treated with vehicle or diabody (0.8 mg/kg) on reperfusion (60 minutes postocclusion). All parameters were measured 24 hours after stroke onset. Data are represented as mean ± SEM (panels B,F) or median (panels C-D); n = 10-12 mice per group (panels B-D); n = 4 mice per group (panel F). *P < .05; **P < .01.
Thrombolytic effect of the diabody on fibrin-rich clots in a model of thrombin-induced stroke
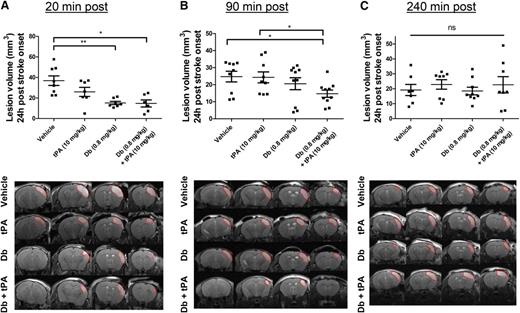
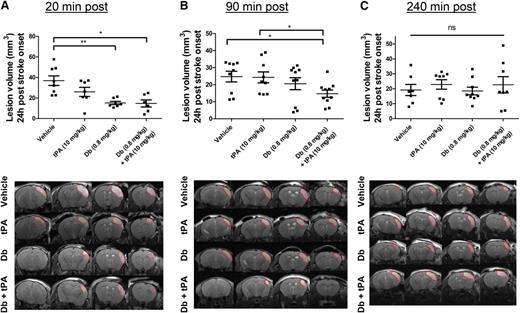
A model of thromboembolic stroke by local thrombin injection was used in which clots are rich in fibrin and thus susceptible to be thrombolysed by tPA when administered early after clot onset; that is, at 20 minutes postocclusion. The efficacy of the diabody was compared to that of tPA, the current thrombolytic agent. To mimic the clinical procedure of thrombolysis, the administration of tPA was performed by an initial bolus of 10% volume, followed by 90% infusion during 40 minutes because of the short half-life of circulating tPA (∼5 minutes35 ). At 24 hours postocclusion, complete recanalization of the arterial lumen occurred in all groups, including the vehicle group (median angiographic score = 2). Lesion volume was reduced by early administration of tPA; however, this reduction was not statistically significant (37 ± 13 mm3 [vehicle] vs 26 ± 12 mm3 [t-PA]; Figure 3A; P = .203; n = 6-8 mice per group). In contrast, early diabody administration (0.8 mg/kg), regardless of the coadministration of tPA, substantially reduced lesison volume at 24 hours (15 ± 4 mm3 [diabody] and 15 ± 8 mm3 [diabody plus tPA]; P < .01 and P < .05 vs vehicle, respectively; n = 6-8 mice per group; Figure 3A).
Evaluation of the thrombolytic efficacy of the diabody on fibrin-rich clots in a model of thrombin-induced MCAo. Lesion volume at 24 hours postocclusion (top) and representative T2-weighted images at 24 hours postocclusion (bottom) of mice treated with vehicle, tPA (10 mg/kg), diabody (0.8 mg/kg), or a combination of diabody (0.8 mg/kg) and tPA (10 mg/kg) at 20 minutes postocclusion (n = 6-8) (A); at 90 minutes postocclusion (n = 9-10) (B); and at 240 minutes postocclusion (n = 7-9) (C). Dotted red lines delineate stroke lesions. Data are represented as mean ± SEM. *P < .05; **P < .01. ns, not significant.
Evaluation of the thrombolytic efficacy of the diabody on fibrin-rich clots in a model of thrombin-induced MCAo. Lesion volume at 24 hours postocclusion (top) and representative T2-weighted images at 24 hours postocclusion (bottom) of mice treated with vehicle, tPA (10 mg/kg), diabody (0.8 mg/kg), or a combination of diabody (0.8 mg/kg) and tPA (10 mg/kg) at 20 minutes postocclusion (n = 6-8) (A); at 90 minutes postocclusion (n = 9-10) (B); and at 240 minutes postocclusion (n = 7-9) (C). Dotted red lines delineate stroke lesions. Data are represented as mean ± SEM. *P < .05; **P < .01. ns, not significant.
When delaying the treatments to a clinically more relevant time point; for example, at 90 minutes postocclusion (intermediate time point),36 complete recanalization was also observed at 24 hours postocclusion in all treatment groups (median angiographic score = 2). Neither intermediately delayed administration of diabody nor infusion of tPA had any beneficial effect on the lesion volume (25 ± 3 mm3 [vehicle] vs 24 ± 3 mm3 [tPA] vs 21 ± 4 mm3 [diabody]; Figure 3B; n = 9-10 mice per group). However, at the same treatment time point, diabody administration prior to tPA infusion resulted in a significantly reduced lesion volume (15 ± 2 mm3 [diabody plus tPA]; P < .05 vs vehicle; n = 10 mice per group; Figure 3B).
None of the treatments had an effect on lesion sizes in this model when administered at 240 minutes postocclusion (late time point, Figure 3C).
Assessment of bleeding
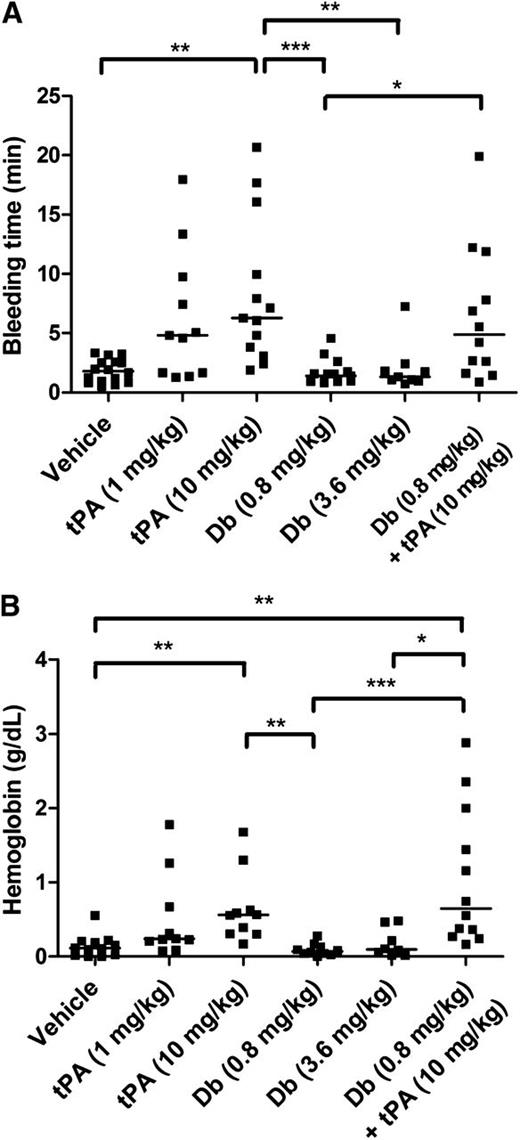
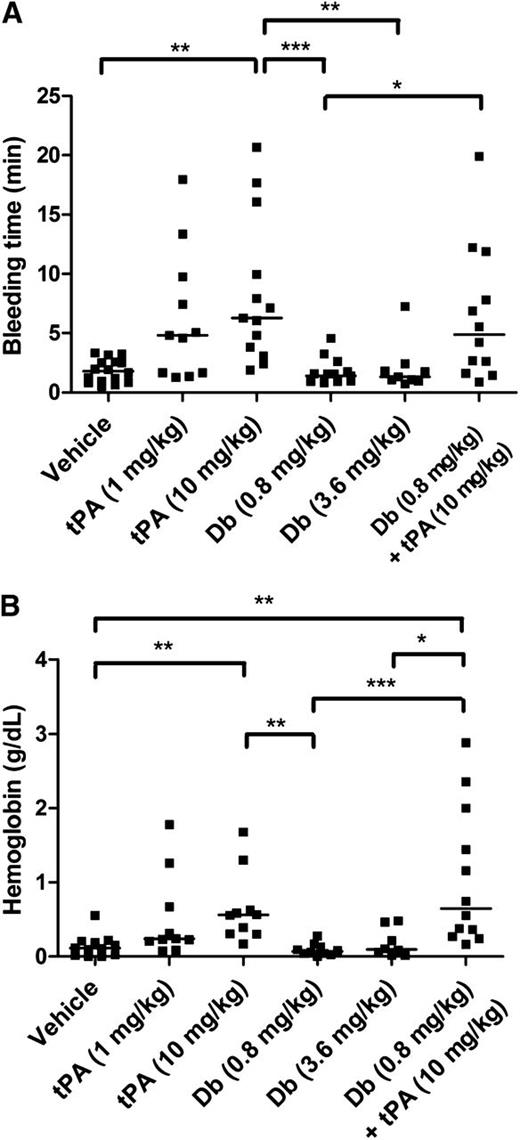
Tail bleeding experiments were performed to compare the effects of tPA administered by IV injection at 2 different doses: the dose equivalent to that used in clinical practice for humans (1 mg/kg) and the dose typically used in mice (10 mg/kg). Diabody was injected at 0.8 mg/kg and 3.6 mg/kg. IV administration of diabody up to 3.6 mg/kg did not alter tail bleeding time or accumulative hemoglobin loss after 60-minute tail incubation, whereas both doses of tPA prolonged bleeding time and increased hemoglobin loss (Figure 4; n = 9-10 mice per group). Coadministration of diabody (0.8 mg/kg) and tPA (10 mg/kg), the treatment regimen tested in the thrombin-mediated MCAo model, did not further increase the tail bleeding time or hemoglobin loss compared to tPA administration alone.
Tail bleeding time and accumulative hemoglobin loss after IV diabody, tPA, or diabody plus tPA. (A) Time until initial cessation of tail bleeding. (B) Accumulative bleeding up to 60 minutes, measured as g/dL hemoglobin. Data are expressed as the median of 9 to16 mice per group. *P < .05; **P < .01; ***P < .005.
Tail bleeding time and accumulative hemoglobin loss after IV diabody, tPA, or diabody plus tPA. (A) Time until initial cessation of tail bleeding. (B) Accumulative bleeding up to 60 minutes, measured as g/dL hemoglobin. Data are expressed as the median of 9 to16 mice per group. *P < .05; **P < .01; ***P < .005.
Alternatively, no cerebral hemorrhages were observed in either mechanical or thrombotic MCAo stroke models after any treatment.
Discussion
A considerable amount of clinical data link elevated levels of antifibrinolytic proteins to a potentiated risk for intravascular thrombosis, recurrent thrombosis, and thrombolytic failure.7,8,10,11 Antifibrinolytic proteins, such as TAFI and PAI-1, have been extensively studied as drug targets in thrombosis-related pathologies.13 Notwithstanding promising results, not all studies proclaim that inhibition of 1 antifibrinolytic constituent is sufficient to overcome thrombosis.31,37,38 Because TAFI and PAI-1 have complementary roles that are localized at the fibrin surface, we hypothesized that simultaneous targeting of the 2 antifibrinolytics would amplify the profibrinolytic capacity in a fibrin-localized manner. Fibrinolysis is driven by enzymatic activation of plasminogen into active plasmin through the exposure of C-terminal lysines subsequent to plasmin-mediated cleavage of fibrin.4 Because these C-terminal lysines serve as cofactors for tPA-mediated activation of plasminogen, this results in a self-propagating dissolution of fibrin. Through the enzymatic removal of C-terminal lysines from fibrin, TAFIa thereby confines fibrinolysis. TAFIa, however, prevents fibrinolysis from entering into the propagation phase only when above a certain threshold concentration, which is proportionate to the level of plasmin activity (ie, a higher level of plasmin generation requires a higher amount of generated TAFIa to withhold fibrinolysis). In terms of a profibrinolytic strategy, a PAI-1 inhibitor leads to increased tPA activity, resulting in more plasmin generation and therefore causing an elevated threshold concentration of TAFIa. The latter in combination with a TAFI inhibitor results in a strong profibrinolytic effect; that is, fibrinolysis occurs in the presence of much lower concentrations of tPA.
To facilitate drug development, a 2-in-1 molecule was designed, called a diabody, to target TAFI and PAI-1 simultaneously. An additional advantage of such a construct was previously exemplified in vitro with Db-T12D11x33H1F739 and Db-TCK26D6x33H1F7.22 Db-TCK26D6x33H1F7 was specifically generated for the species cross-reactive properties of the parental antibodies: in addition to its cross-reactivity toward human TAFI and human PAI-1 allowing its potential clinical use, the diabody can also be evaluated in preclinical studies (without the need of a surrogate diabody). In both in vitro studies, the diabodies were more efficient in increasing fibrinolysis than the combined use of the corresponding parental antibodies. The increased in vitro potency is most likely the consequence of the smaller (58 kDa) and more flexible structure of a diabody, favoring efficient penetration and diffusion into the blood clot. The current study describes the first use of this profibrinolytic strategy in vivo. Db-TCK26D6x33H1F7 was first applied thromboprophylactically in a well-established mouse model of thromboplastin-induced thromboembolism in which a low dose of 0.8 mg/kg (resulting in an equimolar plasma concentration of TAFI) was maximally effective in clearing fibrin from lungs (Figure 1).
A common phenomenon in stroke patients is secondary infarct growth despite initial vessel recanalization. The precise mechanisms of this so-called reperfusion injury are still not completely understood but involve complex thromboinflammatory processes. Intravascular fibrin deposition—trapping erythrocytes, leukocytes, and platelets—has shown to directly obstruct cerebral microvascular perfusion during ischemia and reperfusion.40-43 To further assess the potential profibrinolytic effect of the diabody in this setting, it was evaluated in a mechanical tMCAo model of cerebral ischemia/reperfusion injury. The diabody prominently reduced fibrin(ogen) deposition and lesion volume, which was associated with improved neurologic and motor outcome at 24 hours (Figure 2). In line with this observation, TAFI and PAI-1 levels are increased in patients suffering from ischemic stroke, often correlating with stroke severity, suggesting a negative impact of these antifibrinolytic molecules in stroke development.8,44-50 Interestingly, whereas the diabody ameliorated the outcome in this model, tPA has a controversial effect, potentially through aggravating neuronal damage after focal cerebral ischemia.51 As a control, an in vitro neurotoxicity assay was performed with or without N-methyl-d-aspartate and demonstrated the absence of any neurotoxicity associated with the diabody (supplemental Figure 4).
Because there is an unmet clinical need to optimize current thrombolytic treatment of acute ischemic stroke, we next focused on mouse models of thrombotic MCAo. Current treatment of acute ischemic stroke consists of a high dose of tPA, which is effective only within 4.5 hours after symptom onset; however, only 20% of the small number of patients who arrive in time receive thrombolytic therapy.2,3 This reluctance against thrombolysis stems from life-threatening side effects (hemorrhagic transformation and possible neurotoxicity of tPA) and a thrombus-resolving efficiency that is rather low.52,53 Unexpectedly, neuroprotective agents, which block molecular elaboration of ischemic insult on brain cells, have shown no clinical benefit in patients despite their preclinical efficacy.54 Because recanalization of the occluded vessel is in essence associated with improved clinical outcome,1 it makes sense to target proteins such as TAFI and PAI-1 that inhibit thrombolysis.11 Indeed, when the diabody (0.8 mg/kg) was administered early after thrombin-mediated MCAo, a strong beneficial effect on lesion volume was observed (Figure 3A). This reduction of lesion volume even exceeded the effect of a high systemic dose of tPA (10 mg/kg). To the best of our knowledge, this is the first report of a pharmacologic treatment of fibrin-rich clots resulting in a more efficient lysis than with systemic administration of plasminogen activators.
According to a meta-analysis of 3 stroke trials (Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke, European Cooperative Acute Stroke Study, and National Institute of Neurological Disorders and Stroke rt-PA stroke trial), the greatest benefit is observed when tPA treatment is initiated within 90 minutes after stroke onset.36 At ≥90 minutes after stroke onset, tPA treatment does not always result in a beneficial outcome, presumably because of the increased stability of the clot (ie, clot retraction) resulting in thrombolytic resistance, the neurotoxic effect of tPA to the progressively damaged brain, the increased risk for hemorrhagic transformation, or a combination of these effects. Therefore, in the current study, a 90-minute postocclusion time was tested in the thrombin-mediated MCAo model (Figure 3B). In contrast to the earlier treatment time point (ie, at 20 minutes postocclusion), neither tPA treatment nor diabody treatment at 90 minutes postocclusion had a beneficial effect on the lesion volumes. Interestingly, the combined treatment of the diabody and tPA resulted in a significantly decreased lesion volume, underscoring the potential clinical benefit of adding the diabody to current thrombolytic treatment. Furthermore, a later treatment time point of 4 hours postocclusion was tested, a time point at which tPA was previously established to have had a deleterious effect (ie, increased lesion volume). In our study, however, we observed only a tendency toward increased lesion volumes after tPA treatment, alone or with diabody (Figure 3C). Diabody treatment at this time point did not have any effect, which is in line with the spontaneous resolution of the thrombin-induced clot in this model.55 In correspondence to the in vitro neurotoxicity data (supplemental Figure 4), the diabody also had no deleterious effect in vivo.
To address another clinically relevant issue in the thrombolytic treatment of stroke, a FeCl3-induced MCAo model was used in which platelet-rich clots are formed that are resistant to tPA. The presence of resistant platelet-rich clots contributes to the failure of thrombolytic therapy in the majority of patients.52,53 Thus, in this model, tPA does not reduce the lesion volume nor does it improve recanalization, even when administered early (ie, at 20 minutes postocclusion).29 In clinical practice, cerebral blood flow (CBF) is often measured during and early after tPA administration to evaluate the success of tPA-mediated thrombolysis.56 For this reason, in this model, CBF of the MCA was monitored by laser Doppler flowmetry up to 1 hour after stroke onset in this set of experiments. In addition, recanalization of the MCA was determined by magnetic resonance angiography at 24 hours. As expected, no increase in either CBF or recanalization rate was observed with vehicle or exogenous tPA, whereas the diabody (at 1.6 mg/kg and 3.6 mg/kg) effectuated a slight but significant increase of CBF at 1 hour, which further led to an increased recanalization rate at 24 hours (supplemental Figure 3). Although no difference was detected between both doses of diabody in terms of CBF and recanalization rate, only the higher dose was able to decrease lesion volumes.
Bleeding is a feared complication of thrombolysis, especially in the cerebral circulation. In the thrombosis-induced stroke models, no cerebral bleeding was observed by MRI. However, these models are not suitable for excluding possible cerebral bleeding, because even after tPA administration, no hemorrhagic transformations occurred. Cerebral bleeding tendency is related to the lesion volume, which is relatively small in these models. The monofilament, however, blocks the whole MCA-perfused territory, which typically yields large lesions and more easily transforms into cerebral bleeding. In this model, tPA has been previously demonstrated to increase the incidence of bleeding and edema,57 whereas the diabody in this study did not.
To study the risk of systemic bleeding on traumatic injury, we performed a tail bleeding assay in which tPA and diabody were compared (Figure 4). A similar prolongation of bleeding time on administration of tPA was observed as in a previous study.58 The diabody, however, caused no bleeding time prolongation nor increased accumulative hemoglobin loss. These results are in agreement with a previous study in which TAFI/PAI-1 double-deficient mice showed no prolonged bleeding.31 With TAFI and PAI-1 inhibition, the endogenous tPA activity is increased but most likely does not exceed the systemic antifibrinolytic capacity of α2-antiplasmin. In case of potential side effects, the relatively short half-life of the diabody is also advantageous; however, it is still long enough to allow a bolus administration to obtain sufficiently high levels for several hours.
In conclusion, we have created a strong fibrinolytic enhancer, designated as Db-TCK26D6x33H1F7, with a robust in vivo performance in a variety of thrombotic and stroke mouse models. Our results show that the diabody has potential clinical applications in the treatment of thrombotic disorders as (1) prophylaxis of venous thromboembolism, (2) treatment of brain ischemia/reperfusion injury, and (3) thrombolytic treatment of both fibrin-rich and platelet-rich clots in ischemic stroke. This novel profibrinolytic approach appears to be potentially safer and more effective in mouse stroke models than the current treatment with tPA. On the basis of these results, this diabody holds promise as a candidate for clinical studies of thrombotic disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Maxime Gauberti for his valuable scientific suggestions; and acknowledge Dr Cyrille Orset and Anthony Levilly from the Experimental Stroke Research Platform, University of Caen Lower Normandy, Caen, France.
This study was supported by the National Fund for Scientific Research–Flanders (FWO-Vlaanderen) grants G.0594.13 (P.J.D.) and G.0A86.13N (S.F.D.M.); and by INSERM (D.V.).
Authorship
Contribution: T.W. designed and performed the research, analyzed and interpreted the data, performed statistical analysis, and drafted the manuscript; M.R., F.D., and S.M.d.L. performed the research and analyzed the data; M.P. provided technical assistance; A.G. provided scientific suggestions and contributed to manuscript review; S.F.D.M. and D.V. contributed to the design of the study and to manuscript review; P.J.D. conceived and designed the study, coordinated the experiments, and reviewed the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: A patent application has been filed to protect the intellectual property of the described diabody.
Correspondence: Paul J. Declerck, Laboratory for Therapeutic and Diagnostic Antibodies, Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Campus Gasthuisberg, O&N2, PB 820, Herestraat 49, B-3000 Leuven, Belgium; e-mail:paul.declerck@pharm.kuleuven.be.