Key Points
The Gardos channel is a potassium channel involved in red cell volume modification.
A mutation in KCNN4 encoding the Gardos channel is presented as the genetic basis for a new type of hereditary xerocytosis.
Abstract
The Gardos channel is a Ca2+-sensitive, intermediate conductance, potassium selective channel expressed in several tissues including erythrocytes and pancreas. In normal erythrocytes, it is involved in cell volume modification. Here, we report the identification of a dominantly inherited mutation in the Gardos channel in 2 unrelated families and its association with chronic hemolysis and dehydrated cells, also referred to as hereditary xerocytosis (HX). The affected individuals present chronic anemia that varies in severity. Their red cells exhibit a panel of various shape abnormalities such as elliptocytes, hemighosts, schizocytes, and very rare stomatocytic cells. The missense mutation concerns a highly conserved residue among species, located in the region interacting with Calmodulin and responsible for the channel opening and the K+ efflux. Using 2-microelectrode experiments on Xenopus oocytes and patch-clamp electrophysiology on HEK293 cells, we demonstrated that the mutated channel exhibits a higher activity and a higher Ca2+ sensitivity compared with the wild-type (WT) channel. The mutated channel remains sensitive to inhibition suggesting that treatment of this type of HX by a specific inhibitor of the Gardos channel could be considered. The identification of a KCNN4 mutation associated with chronic hemolysis constitutes the first report of a human disease caused by a defect of the Gardos channel.
Introduction
Hereditary xerocytosis (HX) (Online Mendelian Inheritance in Man 194380) is an autosomal dominant congenital hemolytic anemia characterized by primary erythrocyte dehydration.1 In HX patients, red blood cells exhibit altered intracellular cation content and cellular dehydration, which are responsible for an increased erythrocyte mean corpuscular hemoglobin concentration (MCHC) and a decreased erythrocyte osmotic fragility.2 Under the microscope, blood films show various cell shape abnormalities, the most characteristic being a central pallor, straight or crescent shaped. These cells are called stomatocytes, and HX is also known as dehydrated hereditary stomatocytosis.3
HX has been associated with missense mutations in FAM38A encoding the red cell membrane mechanosensitive cation channel PIEZO1.4,5 Functional studies have demonstrated that in PIEZO1, mutations slowed channel inactivation and introduced a pronounced latency for activation.6 More recently, another type of red cell ion exchange defect associated with pseudohyperkalemia has been linked to mutations in the ATP-binding cassette transporter ABCB6.7
The Gardos channel is a cation channel also referred to as KCa3.1 or KCNN4. It is a Ca2+-sensitive, intermediate conductance, potassium selective channel, initially described in pancreas cells but present in many cell types including erythrocytes.8 A number of recent studies have described its role in a variety of physiologic events and identified it as an interesting therapeutic target in a large panel of human diseases.9,10 We report the first identification of a missense mutation located in one of the functional regions of the Gardos channel and its association with chronic hemolysis and dehydrated cells in 2 unrelated families, with 8 affected persons.
Material and methods
Hematologic tests
An osmotic fragility test in hypotonic saline solutions was performed on red blood cells immediately after sampling and after 24-hour incubation at 4° or 37°C.
Nuclear magnetic resonance
Nuclear magnetic resonance experiments were performed on a 400 AVANCE wide-bore spectrometer (Bruker Biospin, Billerica, MA), using stimulation by the ionophore A23187 (Sigma Aldrich).
Next-generation sequencing
Exome sequencing was performed after exome enrichment using Ion AmpliSeq (Thermo Fisher Scientific, Waltham, MA), template preparation using the Ion PI Template OT2 200 Kit v2 on the Ion OneTouch 2 System, and sequencing using the Ion PI Chip Kit v2 and Ion PISequencing 200 Kit v2 on the Ion Proton Sequencer (Thermo Fisher Scientific). Raw data were first aligned with the provided software suite to generate BAM files. The coverage and sequencing depth analysis were computed using the BEDtools suite v2.1711 and in-house scripts. Variants were identified using the Torrent Browser Variant caller (version 4.0.2), annotated, and prioritized with the in-house VarAFT system that includes Annovar.12
The mutation was confirmed on DNA samples and KCNN4 transcripts from fresh reticulocytes by Sanger sequencing (3500XL Genetic Analyzer; Life Technologies, Carlsbad, CA).
Expression in Xenopus oocytes
Plasmid pcDNA3KCNN4-HA was used to introduce the point mutation p.Arg352His by polymerase chain reaction. A hemagglutinin tag (HA) was present in the C-terminal end of KCNN4.13 Female Xenopus laevis were anesthetized with MS222 according to the procedure recommended by our ethics committee. Oocytes were harvested and injected as previously published.14
Current recording was performed as follows: a ramp protocol between −120 and +80 mV for 2 seconds, holding potential at −80 mV, was applied using Clampex (PClamp; Molecular Devices Corporation). To avoid looking at chloride channel activation, currents were recorded in modified Barth's saline (MBS), where chloride was substituted by gluconate (Na-gluconate, 85 mM; K-gluconate, 1 mM). Junction potential was minimized using an agar bridge and 3 M KCl. Electrodes filled with 3 M KCl had a resistance of 0.5 MOhm. After equilibration in this gluconate MBS, KCNN4 was activated by the calcium ionophore A23187 at 1 µM in MBS gluconate. In control oocytes, no current was activated by ionophore addition.
Western blotting on oocyte
Oocyte membranes were prepared as previously described.15 Immunodetection of KCNN4-HA was done using an anti-HA antibody (1/1000; Sigma). To compare KCNN4 expression levels in different samples, the cell membrane marker β1 Na,K-ATPase was used (1/500; Sigma). Signals were detected by chemiluminescent reaction with Immobilon Western reagent (Millipore) and a Fusion FX7 (Vilber-Lourmat). The intensity of KCNN4 bands relative to the β1 Na,K-ATPase signal was quantified using ImageJ Version 1.44 software (National Center for Biotechnology Information).
HEK293 cell transfection
HEK293 cells were grown in Dulbecco’s modified Eagle medium glutamax (Gibco), 10% fetal bovine serum, and penicillin-streptomycin. Cells were cotransfected with 1 µg WT or point mutated pcDNA3-KCNN4-HA and 0.5 µg plasmid internal ribosome entry site with enhanced yellow fluorescent protein using CaPO4. Sixteen hours later, cells were washed twice with phosphate-buffered saline, and patch clamp was performed on fluorescently labeled cells.
Patch-clamp electrophysiology
Glass pipettes (Brand, Wertheim, Germany) were made on a horizontal pipette puller (P-97; Sutter Instrument, Navato, CA) to give a final resistance ranging from 3 to 5 MΩ. For whole cell experiments, the bath solution was (in mM) NaCl 140, KCl 5, CaCl2 1, glucose 29, and HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) 25, pH 7.4, adjusted with NaOH. The intracellular solution was (in mM) KCl 30, KGluconate 100, EGTA 5, HEPES 10, pH 7.2, adjusted with NaOH, CaCl2 4.19 (corresponding to 1 µM free calcium), and MgATP 2. Currents were measured at room temperature using a ramp protocol from −120 to +80 mV from a holding potential of −60 mV (sampling frequency, 10 kHz; filtered, 1 kHz)
Calcium dependence of KCNN4 was studied with intracellular (bath) solutions (in mM): KCl 30, KGluconate 100, EGTA 5, HEPES 10, pH 7.2 adjusted with KOH, and CaCl2 with varying concentrations 4.91, 4.19, 3.61, 2.82, and 1.7 (10−5, 10−6, 5 × 10−7, 2.5 × 10−7, and 10−7 M of free calcium, respectively). Maxchelator was used to calculate free Ca2+ concentration (http://maxchelator.stanford.edu/CaEGTA-TS.htm). Extracellular solution was (in mM) NaCl 140, KCl 5, CaCl2 1, glucose 29, and HEPES 25, pH 7.4, adjusted with NaOH. Currents were evoked by voltage ramps from −120 to 80 mV (150 ms), filtered at 1 kHz, and acquired with a sampling frequency of 10 kHz. All traces were corrected for liquid junction potential. For dose-response experiments, normalized values of currents at −45 mV were plotted against free Ca2+ concentration.
All patch-clamp experiments were performed with a PC-controlled EPC 9 patch-clamp amplifier (HEKA, Lambrecht/Pfalz, Germany). Currents were acquired and analyzed with Pulse and Pulsefit software (HEKA).
Immunohistochemistry
Immunodetection of KCNN4-HA in HEK293 cells was performed using anti-HA antibody (Sigma-Aldrich).
Red cell cation content and volume measurements
Fresh venous blood was obtained by venipuncture from an informed patient from family 1 and a healthy volunteer. For 24-hour incubation, blood samples were stored at 37°C or 4°C.
For vanadate experiments, blood was washed 4 times at room temperature in medium containing (in mM) NaCl 147, KCl 5, MgSO4 2, CaCl2 1, and HEPES/NaOH 10, pH 7.4. The red cell suspension was then incubated at 37°C and 30% hematocrit, and 5 mM vanadate was added alone or with 10 µM TRAM34. A few minutes before sampling time, 400 µL cell suspension was taken to fill 3 nylon tubes that were centrifuged for 10 minutes at 4°C and 20 000 g at the exact sampling time. The supernatant was collected for extracellular ion content measurements. The pellet of red cells was extracted and immediately weighed. Then, dry weight was measured after overnight heating (80°C). Water content was calculated with a correction of 3.64%, corresponding to trapped medium between packed cells. Intracellular ions were extracted from dried pellets by overnight incubation at 4°C in 5 mL milliRho water (Millipore). Na+ and K+ were measured by flame spectroscopy with an Eppendorf ELEX6361.
Results
We initially investigated a fetus for severe in utero anemia without edema, requiring 1 transfusion in utero at week 27 (hemoglobin [Hb], 30 g/L). After preterm birth, he received 3 additional transfusions, immediately after birth, at 2 weeks (Hb, 65 g/L), and at 6 weeks of age (Hb, 70 g/L), and was then treated with erythropoietin for 6 weeks. Under treatment, the reticulocyte count progressively increased, and Hb value stabilized at 90 g/L at 3 months of age. No further transfusion was necessary. Currently, at 4 years and 10 months of age, the proband demonstrated mild anemia and splenomegaly. A clinical history revealed that the proband’s mother was affected with chronic moderate hemolytic anemia of unknown origin from childhood. She was treated with a regular transfusion regimen from infancy to adolescence. Chelation therapy was started at 8 years of age, and a splenectomy was performed at 25 years old. During her adult life, she received 2 transfusions: one after a delivery and another one during an infection by the parvovirus B19. Four other members of this family originated from France and were also affected by chronic hemolytic anemia (Figure 1A). Three of 4 were splenectomized, and 2 of them have received regular transfusions and chelation therapy for periods of time. To date, none of them presented thrombotic complications.
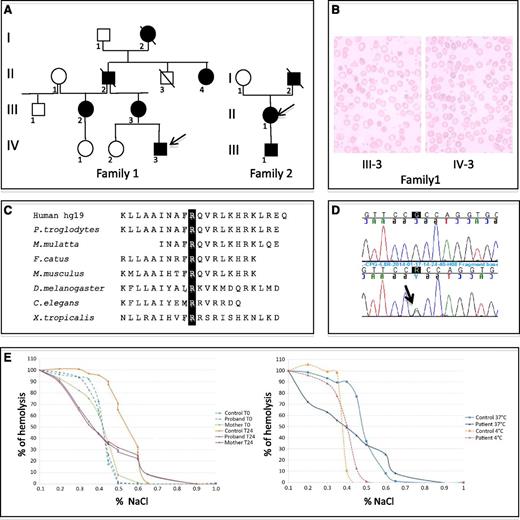
Red blood cell and DNA investigations. (A) Family pedigrees showing mutation segregation. (B) Blood film smears (MGG) for proband 1 and his mother. (C) Multiple interspecies protein sequence alignment of KCNN4 in the region of residue 352. (D) KCNN4 transcript sequencing: (upper) WT sequence and (lower) transcript with mutation c.1055G>A (p.Arg352His). (E) Red cell osmotic fragility test using osmotic gradient ranging from 0.1% to 1% of NaCl solution. (Left) At T0 and at T24-hour incubation at 37°C for a control and individuals III-3 and IV-3 from family 1. (Right) After T24-hour incubation at 4°C or 37°C for a control and individual III-2 from family 1.
Red blood cell and DNA investigations. (A) Family pedigrees showing mutation segregation. (B) Blood film smears (MGG) for proband 1 and his mother. (C) Multiple interspecies protein sequence alignment of KCNN4 in the region of residue 352. (D) KCNN4 transcript sequencing: (upper) WT sequence and (lower) transcript with mutation c.1055G>A (p.Arg352His). (E) Red cell osmotic fragility test using osmotic gradient ranging from 0.1% to 1% of NaCl solution. (Left) At T0 and at T24-hour incubation at 37°C for a control and individuals III-3 and IV-3 from family 1. (Right) After T24-hour incubation at 4°C or 37°C for a control and individual III-2 from family 1.
In a second unrelated family (family 2; Figure 1A), the index case was a 25-year-old woman who has had moderate chronic hemolytic anemia since early childhood. She was never transfused and underwent a cholecystectomy because of biliary lithiasis. Her father originated from Poland and was reported to have severe hemolytic anemia treated by splenectomy and occasional transfusions. Her 2-year-old son was born after a normal pregnancy carried to term, and he also presented with well-tolerated chronic hemolytic anemia. The hematologic parameters of individuals IV-3 and III-3 from family 1 and II-1 and III-3 from family 2 are summarized in Table 1. In addition to anemia, all 4 have a discrete increase in MCHC value.
A microscopic examination of blood smears from individual IV-3 showed mild anisopoikilocytosis with <1% of target cells, polychromatophilic red blood cells, teardrop cells, elliptocytes with sometimes abnormal hemoglobin distribution, hemighosts, bite cells, knizocytes, schizocytes, and rare stomatocytic red cells (Figure 1B). For his mother and for individual II-1 from family 2, anomalies were similar, with more significant anisopoikilocytosis and the presence of acanthocytes (Figure 1B). There was no basophilic stippling of red blood cells.
The eosin 5′maleimide test, electrophoresis of red cell membrane proteins, and hemoglobin study were normal for all of them. The diagnosis of xerocytosis was not retained initially as there was almost no stomatocytes on blood films, and repeated ektacytometry was considered normal for all 4 affected individuals tested.
Whole exome sequencing was performed for 3 subjects in family 1, the proband IV-3, his affected mother III-3, and his unaffected sister IV-2. Variants were filtered against dbSNP137 and for heterozygous exonic mutations present in only the affected individuals. Thirty-three genes were found carrying exonic, nonsynonymous heterozygous mutations, among which KCNN4 encoding the Gardos channel was the most consistent candidate because of its expression in red cells. The missense mutation c.1055G>A (p.Arg352His), confirmed by Sanger sequencing, is located in the Calmodulin interacting region and involves a residue that is highly conserved among species (Figure 1C); it was predicted pathogenic by in silico analysis (Polyphen: http://genetics.bwh.harvard.edu/pph2), with a score of 0.992 from a maximum of 1. Mutation segregation was studied in 3 other members of family 1, 2 affected and 1 unaffected by chronic hemolysis, and was consistent with a dominant transmission of the phenotype linked to the mutation. By direct sequencing of KCNN4 in the 2 affected subjects of family 2, we identified the same missense mutation c.1055G>A in the heterozygous condition for both of them. Splicing was not affected by the substitution, as only normal-sized transcripts were found (Figure 1D). Using the data of exome sequencing in individual IV-3, we confirmed that no pathogenic mutation was present in FAM38, encoding PIEZO1 and described as the major cause of HX until now.
Further investigations were then performed for individual IV-3 and his mother. Osmotic fragility was tested to check red cell dehydration. Both mother and son had an abnormal profile after 24 hours of incubation at 37°C: 50% red cell lysis was obtained with reduced salt concentration compared with a normal control (Figure 1E). The profiles were similar to a normal control when the same analysis was performed after 24 hours at 4°C, explaining why ektacytometry was normal as it was systematically performed after incubation at 4°C. Plasmatic K+ concentrations in various conditions of time and temperature after sampling were measured by potentiometry and were in normal ranges. Dynamic efflux of K+ under Ca2+ stimulation was assessed by 39K NMR of erythrocytes suspensions using stimulation by the ionophore A23187. Except for a short delay in K+ exit following Ca2+ activation, no perturbation was observed (data not shown).
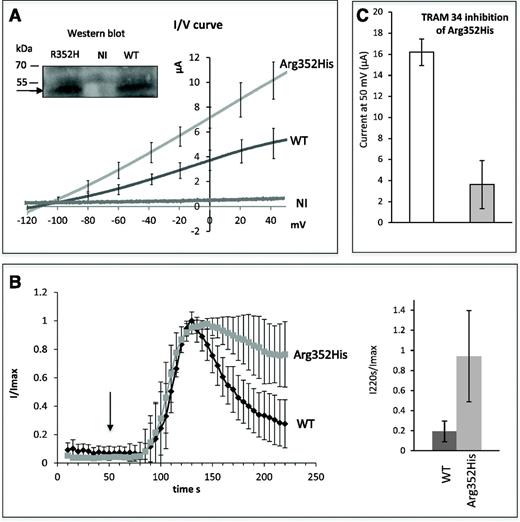
The function of the Gardos channel variant p.Arg352His was then investigated by the expression of a plasmid containing the mutated cDNA in X laevis oocytes. The current voltage curves showed that the missense mutation p.Arg352His does not prevent ion conductance through the channel (Figure 2A). The reversal potential (−120 mV) was similar between WT and the mutated channel, but the current elicited by p.Arg352His KCNN4 expression in Xenopus oocytes was higher than observed with WT. Western blots confirmed that both proteins are expressed at similar levels, suggesting that the conductance increase observed for the mutated channel is directly associated with the mutation. The activation phase of the WT and p.Arg352His channel induced by calcium ionophores were similar (Figure 2B), but, whereas WT KCNN4 activity decreased after reaching a maximum, the p.Arg352His mutant activity remained quite constant for several minutes. The high and sustained current with p.Arg352His made it difficult to record for >2 minutes after the peak. These data indicate that the mutation alters the regulation of channel activity favoring a longer activated state. In parallel, inhibition tests performed with TRAM34, the classical inhibitor of KCNN4, resulted in decreased current production, indicating that the p.Arg352His variant is sensitive to inhibition (Figure 2C).
Functional analysis of the Gardos channel variant p.Arg352His. (A) Current-voltage curves of WT and mutated KCNN4 in oocyte membranes with quantification. I/V curves correspond to the maximal current recorded for WT or p.Arg352His KCNN4-expressing oocytes (around 135 seconds) in gluconate medium with 1 µM A23187. Data are means of ramps recorded on 15 (WT) or 22 (p.Arg352His) oocytes. NI are control (noninjected) oocytes (n = 4). (Inset) Western blot detection of WT and mutated KCNN4 indicated by the arrow (around 50 kDa). (B) Activation kinetic. For oocytes expressing WT KCNN4 or p.Arg352His KCNN4, the current measured in gluconate medium at 0 mV was plotted as a function of time (left). The maximal intensity of the current being different between WT and mutated KCNN4, a ratio between I at different times and the Imax was calculated for each condition and plotted as a function of time. Data are means of 15 (WT) or 22 (p.Arg352His) oocytes coming from 3 different batches. The arrow indicates the opening of calcium ionophore perfusion. The addition of A23187 did not stimulate any current in noninjected oocytes; for clarity, the NI trace was not plotted on this graph. The bar graph (right) quantifies the remaining current at 220 seconds in oocytes expressing WT or p.Arg352His KCNN4. The current at 220 seconds was divided by Imax (at about 135 seconds) for each recording. Data are means ± standard error of the mean (SEM) of 15 (WT) or 22 (p.Arg352His) oocytes. Statistical analysis was done using the Mann-Whitney test; the 2 bars are different with a risk of 0.2% (bidirectional). (C) TRAM34 inhibition: once the maximal current was reached in oocytes expressing p.Arg352His mutant, 10 µM TRAM34 was added. This induced a rapid current decrease. The mean value of maximal currents at 50 mV was calculated (white bar) and compared with the mean value of minimal currents at 50 mV after TRAM34 addition (gray bar). Data are means of 4 oocytes ± SEM.
Functional analysis of the Gardos channel variant p.Arg352His. (A) Current-voltage curves of WT and mutated KCNN4 in oocyte membranes with quantification. I/V curves correspond to the maximal current recorded for WT or p.Arg352His KCNN4-expressing oocytes (around 135 seconds) in gluconate medium with 1 µM A23187. Data are means of ramps recorded on 15 (WT) or 22 (p.Arg352His) oocytes. NI are control (noninjected) oocytes (n = 4). (Inset) Western blot detection of WT and mutated KCNN4 indicated by the arrow (around 50 kDa). (B) Activation kinetic. For oocytes expressing WT KCNN4 or p.Arg352His KCNN4, the current measured in gluconate medium at 0 mV was plotted as a function of time (left). The maximal intensity of the current being different between WT and mutated KCNN4, a ratio between I at different times and the Imax was calculated for each condition and plotted as a function of time. Data are means of 15 (WT) or 22 (p.Arg352His) oocytes coming from 3 different batches. The arrow indicates the opening of calcium ionophore perfusion. The addition of A23187 did not stimulate any current in noninjected oocytes; for clarity, the NI trace was not plotted on this graph. The bar graph (right) quantifies the remaining current at 220 seconds in oocytes expressing WT or p.Arg352His KCNN4. The current at 220 seconds was divided by Imax (at about 135 seconds) for each recording. Data are means ± standard error of the mean (SEM) of 15 (WT) or 22 (p.Arg352His) oocytes. Statistical analysis was done using the Mann-Whitney test; the 2 bars are different with a risk of 0.2% (bidirectional). (C) TRAM34 inhibition: once the maximal current was reached in oocytes expressing p.Arg352His mutant, 10 µM TRAM34 was added. This induced a rapid current decrease. The mean value of maximal currents at 50 mV was calculated (white bar) and compared with the mean value of minimal currents at 50 mV after TRAM34 addition (gray bar). Data are means of 4 oocytes ± SEM.
To further characterize the p.Arg352His KCNN4, HEK293 cells were transfected with the WT or mutated channel. The mutation did not prevent the addressing of the channel to plasma membrane as illustrated on Figure 3A-B. Whole cell recording showed a different calcium-dependent activation kinetic for HEK293 cells expressing WT or p.Arg352His KCNN4 (Figure 3B). For the former, the current progressively appeared while Ca2+ was diffusing from the pipette to the intracellular compartment. By contrast, in the latter case, the current was activated immediately after break-in for the mutant and further increased during the time of recording. As in oocyte experiments, the maximum current density was increased in p.Arg352His KCNN4-expressing cells (Figure 3B-D). These results suggest that the mutation increases the sensitivity of the channel to calcium. The leftward shift in reversal potential observed between break-in and steady state for WT confirms the delay due to Ca2+ diffusion to activate the channel. This delay is not observed for p.Arg352His KCNN4. The calcium sensitivity of WT vs mutated KCNN4 was further explored by performing giant excised inside-out patch-clamp experiments. Figure 3E shows representative traces of K+ currents as a function of voltage and Ca2+ concentrations applied to the internal face of the membrane. In Figure 3F, currents at −45 mV are plotted as a function of Ca2+ concentrations. Quantitative analysis showed the calcium dependence of the WT KCNN4 to have an apparent Kd of 0.95 ± 0.09 µM (which is comparable to measurements done on intact red cells16 ), whereas the apparent Kd is 0.21 ± 0.02 µM for p.Arg352His mutant. The Hill coefficients were not statistically different between WT and mutant KCNN4 (3.75 ± 1.45 and 3.3 ± 0.85, respectively, n = 4).
KCNN4 expression in HEK293 cells. (A) Immuno-staining showing expression of WT and mutated KCNN4 at the plasma membrane. WT SK4-HA or SK4 p.Arg352His-HA were transfected in HEK293 cells and stained with an anti-HA antibody (Red). Nuclei were stained with Hoechst (blue). Images are representative of 3 independent experiments. Scale bars, 10 µm. (B) Activation kinetic of WT and p.Arg352His KCNN4 recorded in whole cell configuration. WT KCNN4 or p.Arg352His KCNN4 was expressed in HEK293 cells and then subjected to patch-clamp experiments in whole cell configuration. Currents were recorded immediately after break-in using a 150-ms voltage ramp protocol from −120 to + 80 mV from a holding potential of −60 mV. The current at −20 mV was plotted as a function of time. Values are mean ± SEM of 8 to 12 experiments. (C) Representative current/voltage curves for HEK293 expressing WT or p.Arg352His KCNN4. Inset (upper left) represents reversal potentials just after break-in and at the steady state. Values are represented as a Tukey’s plot (n = 8-12). Statistical analyses were done using the Kruskal-Wallis test followed by a Tukey post hoc test. (D) Tukey’s plots showing current density at −20 mV in WT and mutated SK4 (n = 8-12; ***P < .001). Statistical analysis was performed using a Mann-Whitney test. (E) Representative traces of Ca2+-dependent activation of WT and p.Arg352His KCNN4 current recorded in an inside-out macropatch configuration. Currents were elicited by 150-ms voltage ramps from −120 to + 80 mV. Each trace corresponds to a different concentration of Ca2+ indicated on the right side of the I/V (in micromolar). (F) Normalized K+ current measured at −45 mV in response to [Ca2+]i was plotted as a function of [Ca2+]I for WT (black squares) and p.Arg352His-mutated KCNN4 (red circles). The experimental values (mean ± SEM) were fitted with the Hill equation (Origin Software, Northampton, MA):  , where Y is the relative KCNN4 current at −45 mV (I/Imax) for each [Ca2+], Ymax is the maximum current (Imax), K0.5 is the apparent dissociation constant, and n is the Hill coefficient. Inset (lower right) shows Hill equation parameters K0.5 and nh. Values are represented as a Tukey plot (n = 4; *P < . 05). Statistical analysis was performed using a Mann-Whitney test.
, where Y is the relative KCNN4 current at −45 mV (I/Imax) for each [Ca2+], Ymax is the maximum current (Imax), K0.5 is the apparent dissociation constant, and n is the Hill coefficient. Inset (lower right) shows Hill equation parameters K0.5 and nh. Values are represented as a Tukey plot (n = 4; *P < . 05). Statistical analysis was performed using a Mann-Whitney test.
KCNN4 expression in HEK293 cells. (A) Immuno-staining showing expression of WT and mutated KCNN4 at the plasma membrane. WT SK4-HA or SK4 p.Arg352His-HA were transfected in HEK293 cells and stained with an anti-HA antibody (Red). Nuclei were stained with Hoechst (blue). Images are representative of 3 independent experiments. Scale bars, 10 µm. (B) Activation kinetic of WT and p.Arg352His KCNN4 recorded in whole cell configuration. WT KCNN4 or p.Arg352His KCNN4 was expressed in HEK293 cells and then subjected to patch-clamp experiments in whole cell configuration. Currents were recorded immediately after break-in using a 150-ms voltage ramp protocol from −120 to + 80 mV from a holding potential of −60 mV. The current at −20 mV was plotted as a function of time. Values are mean ± SEM of 8 to 12 experiments. (C) Representative current/voltage curves for HEK293 expressing WT or p.Arg352His KCNN4. Inset (upper left) represents reversal potentials just after break-in and at the steady state. Values are represented as a Tukey’s plot (n = 8-12). Statistical analyses were done using the Kruskal-Wallis test followed by a Tukey post hoc test. (D) Tukey’s plots showing current density at −20 mV in WT and mutated SK4 (n = 8-12; ***P < .001). Statistical analysis was performed using a Mann-Whitney test. (E) Representative traces of Ca2+-dependent activation of WT and p.Arg352His KCNN4 current recorded in an inside-out macropatch configuration. Currents were elicited by 150-ms voltage ramps from −120 to + 80 mV. Each trace corresponds to a different concentration of Ca2+ indicated on the right side of the I/V (in micromolar). (F) Normalized K+ current measured at −45 mV in response to [Ca2+]i was plotted as a function of [Ca2+]I for WT (black squares) and p.Arg352His-mutated KCNN4 (red circles). The experimental values (mean ± SEM) were fitted with the Hill equation (Origin Software, Northampton, MA):  , where Y is the relative KCNN4 current at −45 mV (I/Imax) for each [Ca2+], Ymax is the maximum current (Imax), K0.5 is the apparent dissociation constant, and n is the Hill coefficient. Inset (lower right) shows Hill equation parameters K0.5 and nh. Values are represented as a Tukey plot (n = 4; *P < . 05). Statistical analysis was performed using a Mann-Whitney test.
, where Y is the relative KCNN4 current at −45 mV (I/Imax) for each [Ca2+], Ymax is the maximum current (Imax), K0.5 is the apparent dissociation constant, and n is the Hill coefficient. Inset (lower right) shows Hill equation parameters K0.5 and nh. Values are represented as a Tukey plot (n = 4; *P < . 05). Statistical analysis was performed using a Mann-Whitney test.
According to electrophysiologic data, KCNN4 should be activated by lower calcium concentration in patient red cells compared with controls. To assess the effect of an increase in intracellular Ca2+ on the kinetic of Gardos channel activation in control or patient red cells, the net potassium flux was measured in red cells treated by vanadate. Vanadate increases intracellular Ca2+ concentration in red cells by inhibiting the calcium pump and also by activating the calcium influx.17,18 Figure 4 illustrates the K+ content of control or patient red cells (individual III-2) in the presence of 5 mM vanadate with or without 10 µM TRAM34. Whereas vanadate did not significantly change intracellular K+ content in control red cells over 1 hour, a significant decrease in intracellular K+ was observed in patient red cells, and this decrease was blocked by TRAM34. The K+ efflux was correlated to cell volume decrease as illustrated on Figure 4B. No significant change in Na+ contents was observed in control or in patient red cells at the same time (Figure 4C).
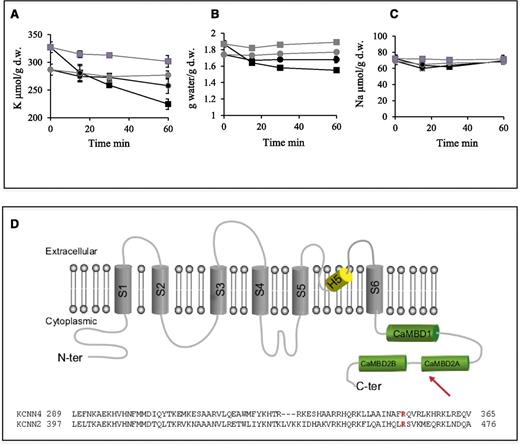
Red cell cation content and volume as a function of incubation time with vanadate. (A) K+ content, (B) cell water, and (C) Na+ content in red cells incubated with 5 mM vanadate (black circles for control red cells; black squares for patient red cells) or 5 mM vanadate with 10 µM TRAM34 (gray circles for control red cells and gray squares for patient red cells). Data are in µmoles per gram of dry weight and are mean ± standard deviation. n = 3. (D) (Upper) On the scheme of the Gardos channel subunit (adapted from Morales et al24 and UniProt accession O15554), the arrow shows the position of the mutated residue and the 3 boxes in green represent the domain interacting with Calmodulin: the Calmodulin is constitutively bound to the 312 to 329 segment of the channel C-terminal region (CaMBD1); the Ca2+-dependent binding of the Calmodulin to the channel involves the segment 344 to 353 (CaMBD2A) and a stretch of 13 amino acids from 360 to 373 (CaMBD2B). (Lower) Protein sequence alignment between KCNN4 and KCNN2 in the region of the CaMBD2A.
Red cell cation content and volume as a function of incubation time with vanadate. (A) K+ content, (B) cell water, and (C) Na+ content in red cells incubated with 5 mM vanadate (black circles for control red cells; black squares for patient red cells) or 5 mM vanadate with 10 µM TRAM34 (gray circles for control red cells and gray squares for patient red cells). Data are in µmoles per gram of dry weight and are mean ± standard deviation. n = 3. (D) (Upper) On the scheme of the Gardos channel subunit (adapted from Morales et al24 and UniProt accession O15554), the arrow shows the position of the mutated residue and the 3 boxes in green represent the domain interacting with Calmodulin: the Calmodulin is constitutively bound to the 312 to 329 segment of the channel C-terminal region (CaMBD1); the Ca2+-dependent binding of the Calmodulin to the channel involves the segment 344 to 353 (CaMBD2A) and a stretch of 13 amino acids from 360 to 373 (CaMBD2B). (Lower) Protein sequence alignment between KCNN4 and KCNN2 in the region of the CaMBD2A.
The K+ content of red cells in blood stored for 24 hours at 37°C or 4°C is given in Table 2. In control red cells, the K+ content is decreased by 21.1 µmol/g dry weight after 24 hours at 37°C. This variation is similar for blood stored for 24 hours at 4°C (−24.1 µmol/g dry weight). In contrast, there is a K+ loss of 93.8 µmol/g dry weight in patient red cells stored at 37°C compared with 35.3 µmol/g dry weight for patient blood stored for 24 hours at 4°C.
Discussion
Water and solute homeostasis is essential for the maintenance of erythrocyte integrity and is controlled via the regulation of monovalent cation content. Several primary disorders of erythrocyte hydration exist and are characterized by an abnormal permeability of the erythrocyte membrane to sodium and potassium, resulting either in swelling or shrinkage of red cells.19 Clinically, these inherited disorders are associated with chronic hemolytic anemia and are due to defects in various transmembrane ion channels or transporters.3
The Gardos channel, a K+ channel, is made of 4 identical subunits; each subunit is encoded by a single gene, KCNN4, and comprises 6 transmembrane domains and a pore region between the fifth and the sixth transmembrane domains (Figure 4D).8 In steady-state conditions, the Gardos channel is inactive. Its function is not fully elucidated in mature normal erythrocytes, but it is probably one of the major players in red blood cell dehydration.20 Under external stimulation, intracellular Ca2+ increases and then interacts with Calmodulin molecules that are bound tightly on each of the 4 channel subunits of the Gardos channel. Ca2+ binding to Calmodulin results in the opening of the channel and rapid K+ and water efflux leading to erythrocyte dehydration and shrinkage, a mechanism referred to as the Gardos effect.8,21 Red blood cells are in constant movement during blood circulation where they experience mechanical stress on their membrane. Using on-cell patch-clamp experiments, it has been shown that local membrane deformation can act as a stimulating event in red cells and lead to Gardos activation, suggesting that this mechanosensory mechanism may allow erythrocytes to adapt their volume and shape to pass through the narrow capillaries of the microvasculature.22
In the present study, the functional experiments performed on Xenopus oocytes showed that the channel mutated on residue 352 is normally activated by Ca2+ influx but permits an increased efflux of K+ compared with the WT channel and remains open and active during a prolonged period. It is likely that the mutation, which removes a positive charge in the Calmodulin binding domain of the Gardos channel, modifies interactions with this activating partner, resulting in a more active channel. Interestingly, Arg352 has already been shown to play a crucial role in the regulation of KCNN4 activity through Calmodulin interaction in 2 different studies. First, in an analysis of the crystal structure of Calmodulin bound to the Calmodulin binding domain (CaMBD) of KCNN2, another Ca2+-activated potassium channel, it is observed that the residue Arg464 in CaMBD interacts with Glu84 in the C-lobe of Calmodulin and is involved in the Ca2+ independent interactions between the CaMBD and Calmodulin.23 This Arg464 residue corresponds to Arg352 in KCNN4 (Figure 4D). Second, in an extensive study analyzing the contribution of the KCNN4-Calmodulin interactions to the regulation of the KCNN4 gating process, it is shown that the interaction between 2 KCNN4 monomers involves electrostatic interactions of Arg352 from 1 monomer with Glu363 of the adjacent monomer and that electrostatic interactions at Glu363 contribute to control the open probability of KCNN4.24 Furthermore, in the same model, the substitution of Arg352 by Cys increases KCNN4 deactivation time. It is thus likely that Arg352 substitution by His also changes electrostatic interactions with Glu363 and therefore affects KCNN4 kinetic properties leading to a prolonged activated state of p.Arg352His KCNN4 as it is observed in our experiments involving this mutant channel.
Experiments on the human cell line HEK293 confirmed the higher current density for mutated KCNN4. This high current density could result from changes in the open probability, in the trafficking, or in the unitary conductance of the channel. The association Calmodulin-KCNN4 is required for the trafficking and assembly of the functional channels in CHO cells.25 Despite the fact that the Calmodulin binding site is mutated, the trafficking properties of the mutant are unaffected in cells with very different trafficking properties (HEK293 and Xenopus oocytes). Thus, the mutation is proposed to affect ion channel functional properties rather than its trafficking. Finally, these experiments showed that p.Arg352His mutation changes the Ca2+ sensitivity of the channel that is activated by 10 times lower Ca2+ concentration.
The anomaly in the kinetic of activation combined with a higher sensitivity to Ca2+ confers pathogenicity to p.Arg352His KCNN4. This is reminiscent of the pathogenic mechanism associated with several of the mutations affecting PIEZO1. Indeed, 3 different studies report that mutations in PIEZO1 are associated with a slowing of inactivation kinetics of this channel.5,6,26
Dehydrated red cells are usually associated with hemolytic anemia because shrinkage stimulates phosphatidylserine exposure as previously shown in both normal red blood cells, glucose-6-phosphate–deficient cells, and hemoglobin S cells.27,28 A relationship between cation leakage and hemolytic anemia has also been observed for other membrane proteins mutations including Band 3 mutations.29 Just like in patients with PIEZO1 dysfunction, we observed in the patients with mutation in the Gardos channel, a variability in disease severity, with a level of anemia rather severe in family 1, whereas individuals of family 2 present normal or subnormal Hb levels.30,31 Furthermore, the clinical pattern of expression is similar in the 2 types of channel defects with an expression in erythroid cells only, whereas both channels are expressed in various tissues. This could be explained by the fact that volume is crucial for a circulating cell such as the red cell which has to face circulatory shear stress associated with changes in oxygenation/deoxygenation. In addition, this point could shed light on PIEZO1 function in erythrocyte, suggesting that PIEZO1 and the Gardos channel might act in the same stretch-induced cation pathway involved in cell volume changes.
In regard to iron overload due to chronic anemia, it is difficult to evaluate in family 1 as individual III-3 has regularly been transfused and treated by deferriprox, and IV-3 is too young to have iron overload. In the second family, the patients exhibit moderate iron overload as observed in chronic hemolytic anemia.
This report will probably lead to additional descriptions of this disorder, which is difficult to diagnose because biologic tests are only slightly altered, although the anemia can be severe. The fact that the osmotic resistance test reveals abnormal profiles only after 24-hour incubation at 37°C is of importance for the diagnosis procedure but also for the mechanistic investigation of the pathogenic effect. Indeed, it suggests that ATP, which is consumed at 37°C but not at 4°C, has to be depleted for abnormal red cell resistance to be observed. At 37°C, the Na+/K+ ATPase and the Ca2+ pump are acting to maintain low intracellular calcium concentration and Na+ and K+ gradients, consuming ATP. With time, ATP is depleted, pump efficiency decreases, and intracellular calcium increases. In the patient red cells, a small Ca2+ increase is sufficient to activate the mutated Gardos channel and to induce red cell dehydration as observed in our experiments.
This identification of a KCNN4 mutation associated with chronic hemolysis and dehydrated cells constitutes the first report of a human disease caused by a defect of the Gardos channel. The description of such a molecular defect provides a rationale for suggesting the treatment of this type of HX by specific inhibitors of the Gardos channel such as the Senicapoc, which was tested in the past in a phase 3 study for the treatment of sickle cell disease and was proven, on this occasion, to be nontoxic.32
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the members from the 2 families for their contribution to this study. The pcDNA3KCNN4-HA plasmid was a kind gift of Leonard Kaczmareck’s laboratory (Yale University, New Haven, CT).
Authorship
Contribution: R.R.-M., C.L., E.L., N.D., V.N., M.L., M.B., O.S., P.V., J.-P.D., C. Béroud, D.S., V.P., M.F.-T., H.G., and C. Badens participated in experimental design, execution, and interpretation; H.V., V.L., C.G., and I.T. selected and characterized patients; C.L., H.G., and C. Badens wrote the paper; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Catherine Badens, INSERM UMR_910, Faculté de Médecine, 27 Blvd Jean Moulin, 13385 Marseille Cedex 5, France; e-mail: catherine.badens@univ-amu.fr.
References
Author notes
H.G. and C. Badens contributed equally to this work.

![Figure 3. KCNN4 expression in HEK293 cells. (A) Immuno-staining showing expression of WT and mutated KCNN4 at the plasma membrane. WT SK4-HA or SK4 p.Arg352His-HA were transfected in HEK293 cells and stained with an anti-HA antibody (Red). Nuclei were stained with Hoechst (blue). Images are representative of 3 independent experiments. Scale bars, 10 µm. (B) Activation kinetic of WT and p.Arg352His KCNN4 recorded in whole cell configuration. WT KCNN4 or p.Arg352His KCNN4 was expressed in HEK293 cells and then subjected to patch-clamp experiments in whole cell configuration. Currents were recorded immediately after break-in using a 150-ms voltage ramp protocol from −120 to + 80 mV from a holding potential of −60 mV. The current at −20 mV was plotted as a function of time. Values are mean ± SEM of 8 to 12 experiments. (C) Representative current/voltage curves for HEK293 expressing WT or p.Arg352His KCNN4. Inset (upper left) represents reversal potentials just after break-in and at the steady state. Values are represented as a Tukey’s plot (n = 8-12). Statistical analyses were done using the Kruskal-Wallis test followed by a Tukey post hoc test. (D) Tukey’s plots showing current density at −20 mV in WT and mutated SK4 (n = 8-12; ***P < .001). Statistical analysis was performed using a Mann-Whitney test. (E) Representative traces of Ca2+-dependent activation of WT and p.Arg352His KCNN4 current recorded in an inside-out macropatch configuration. Currents were elicited by 150-ms voltage ramps from −120 to + 80 mV. Each trace corresponds to a different concentration of Ca2+ indicated on the right side of the I/V (in micromolar). (F) Normalized K+ current measured at −45 mV in response to [Ca2+]i was plotted as a function of [Ca2+]I for WT (black squares) and p.Arg352His-mutated KCNN4 (red circles). The experimental values (mean ± SEM) were fitted with the Hill equation (Origin Software, Northampton, MA): , where Y is the relative KCNN4 current at −45 mV (I/Imax) for each [Ca2+], Ymax is the maximum current (Imax), K0.5 is the apparent dissociation constant, and n is the Hill coefficient. Inset (lower right) shows Hill equation parameters K0.5 and nh. Values are represented as a Tukey plot (n = 4; *P < . 05). Statistical analysis was performed using a Mann-Whitney test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/11/10.1182_blood-2015-04-642496/4/m_1273f3.jpeg?Expires=1769860438&Signature=zOXrZhk4nTnVyXCiurv2yrURnK1EVPMm0IxrgeBSU9x02RnAx~69Vt~hfDWHNa6EuRFQv-j2~HPyA9sI6P5gnBAH9QHcGwQEWYxUV-FRaxUnappK1xJMvdvsX4tVcjU9aKnwKGsGN2NlWhQsZvwzyhCOYUqA8ktGGdL0~u8dN~1R9dW38EALS254pQPCJinqUepqcgG8kfxq4ThTM-qKIhOXnbUQyZzxUtXYv2t9uEW65NFHnCL37O7jN5QRoh4kX3CRgZ3X4-5ICM7~d2NE2rSy3TwJ~ymUkG4y3l0SXgI6X2Y4XuVdQl-4Ye17T-juzFxN3N7KC5Xr~w5UnhQ44A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)