Abstract
Catastrophic thrombotic syndromes are characterized by rapid onset of multiple thromboembolic occlusions affecting diverse vascular beds. Patients may have multiple events on presentation, or develop them rapidly over days to weeks. Several disorders can present with this extreme clinical phenotype, including catastrophic antiphospholipid syndrome (APS), atypical presentations of thrombotic thrombocytopenic purpura (TTP) or heparin-induced thrombocytopenia (HIT), and Trousseau syndrome, but some patients present with multiple thrombotic events in the absence of associated prothrombotic disorders. Diagnostic workup must rapidly determine which, if any, of these syndromes are present because therapeutic management is driven by the underlying disorder. With the exception of atypical presentations of TTP, which are treated with plasma exchange, anticoagulation is the most important therapeutic intervention in these patients. Effective anticoagulation may require laboratory confirmation with anti–factor Xa levels in patients treated with heparin, especially if the baseline (pretreatment) activated partial thromboplastin time is prolonged. Patients with catastrophic APS also benefit from immunosuppressive therapy and/or plasma exchange, whereas patients with HIT need an alternative anticoagulant to replace heparin. Progressive thrombotic events despite therapeutic anticoagulation may necessitate an alternative therapeutic strategy. If the thrombotic process can be controlled, these patients can recover, but indefinite anticoagulant therapy may be appropriate to prevent recurrent events.
A 14-year-old, previously healthy young man developed progressive headaches, nausea, and vomiting, culminating in a tonic-clonic seizure several days following a minor knee injury sustained while playing soccer. He was found to have extensive dural sinus thrombosis and started on anticoagulant therapy, but disseminated intravascular coagulation (DIC) and thrombocytopenia developed, limiting efforts to treat him with anticoagulants. Testing for antiphospholipid antibodies and heparin-induced thrombocytopenia (HIT) was negative, and heparin was restarted as the coagulopathy resolved. Despite aggressive efforts, severe brain swelling with herniation developed, and efforts to save him were withdrawn after documenting lack of blood flow above the internal carotid arteries. At autopsy, he had bilateral pulmonary emboli and iliac vein occlusions in addition to extensive intracranial venous thrombosis.
Introduction
Patients presenting with multiple thromboembolic events affecting diverse vascular beds are uncommon and management in the acute setting can be extremely difficult. Affected individuals are often younger; thrombotic events occur simultaneously or within days to weeks and thrombosis frequently involves unusual sites.1-3 Therapeutic management may be complicated by the concomitant presence of hemorrhagic risk factors (eg, thrombocytopenia, DIC) or the need for surgical intervention. Kitchens first referred to this clinical presentation as “thrombotic storm” in 1998, identifying the following characteristic features: (1) an underlying hypercoagulable state is frequently present; (2) a provocative factor, or “trigger,” is frequently associated with initiation of the thrombotic process; (3) new thromboembolic events develop rapidly, particularly if appropriate treatment is delayed; (4) prompt initiation of antithrombotic therapy is essential to achieve a successful outcome; and (5) long-term prognosis is good, if the cycle of thrombosis is interrupted early.1
These catastrophic clinical presentations may occur in patients with a known prothrombotic disorder (eg, antiphospholipid syndrome [APS], cancer) or in a previously healthy individual, as described in the case noted above. Appropriate clinical management requires rapid identification of any associated hypercoagulable states; however, treatment strategies vary and an incorrect decision can delay initiation of appropriate therapy, increasing the risk for serious adverse outcomes for the individual patient. The purpose of this article is to discuss the diagnosis and management of patients presenting with multiple, rapidly progressive thromboembolic events.
What are the “catastrophic thrombotic syndromes”?
Patients with several distinct disorders can present with this clinical phenotype. It is instructive to briefly review these disorders because the initial clinical presentation can be similar, but treatment approaches differ if one of these diseases is identified (Table 1).
Catastrophic antiphospholipid syndrome
APS is defined as the combination of venous or arterial thrombotic events, and/or recurrent pregnancy morbidity, in association with persistent antiphospholipid antibodies (aPLs). A catastrophic variant of APS was first proposed by Asherson in 1992, based on 10 cases from the literature.4 These patients presented with multiple vascular occlusive events, usually affecting small vessels supplying various internal organs, especially the brain, lungs, and kidneys, and presenting over a relatively short period of time. A subsequent review of 50 patients with catastrophic APS confirmed the microvascular thrombotic events, but also found that a significant number exhibited venous thromboembolism (VTE; 30%), peripheral arterial occlusions (12%), cerebral infarction (18%), and other large vessel occlusions.5 A precipitating factor, or “trigger,” often preceded development of the syndrome, including infections, medication changes, surgical procedures, and anticoagulation withdrawal.5 Larger studies have confirmed that this catastrophic variant is rare, occurring in ∼1% of patients with APS.6
An international registry of patients with catastrophic APS, known as the CAPS Registry, was formed by the European Forum on Antiphospholipid Antibodies in 2000.7 Classification criteria were subsequently developed to identify patients with catastrophic APS (Table 2). Pediatric patients exhibit a similar clinical phenotype as adults, but with a higher prevalence of thrombotic events involving the peripheral vasculature.8 Almost half of all patients with catastrophic APS, and >85% of pediatric patients, present with the catastrophic event as their first manifestation of APS.8,9 Patients with catastrophic APS frequently also manifest thrombocytopenia and hemolytic anemia.10 For patients without a prior diagnosis of APS or systemic lupus erythematosus (SLE), distinguishing catastrophic APS from a non-APS–related disorder can be difficult.11,12
Atypical thrombotic thrombocytopenic purpura/thrombotic microangiopathies
Patients with thrombotic thrombocytopenic purpura (TTP) and other thrombotic microangiopathies typically exhibit schistocytes, thrombocytopenia, and organ injury secondary to arteriole and capillary thrombosis.16 Atypical presentations of TTP have been reported, however, which include patients with macrovascular occlusions, such as acute thromboembolic stroke17-19 and acute coronary syndromes.18,20,21 Confounding the issue further, some of these patients do not exhibit the characteristic laboratory findings of a thrombotic microangiopathy initially, which may develop days to weeks after the initial symptomatic presentation.17,21 Approximately two-thirds of these patients have been previously diagnosed with TTP or a thrombotic microangiopathy. Importantly, the delay in appearance of thrombocytopenia and/or the presence of schistocytes can lead to delays in initiating therapy with plasma exchange and corticosteroids.
Occasionally, patients with SLE may present with TTP,20,22 which can be similar to catastrophic APS. The treatment differs, however, in that anticoagulant therapy is not typically administered in patients with SLE and TTP. Although most patients do not have a specific precipitating event, TTP has been reported to occur after infections,23 surgical procedures,24 and in patients with pancreatitis.25 The pattern of thrombotic events and laboratory findings help distinguish TTP from the other catastrophic thrombotic disorders (Table 1).
Heparin-induced thrombocytopenia and thrombosis
HIT is a transient, immune-mediated prothrombotic disorder caused by antibodies that bind to platelet factor 4 (PF4) in complex with heparin or low-molecular-weight heparin (LMWH).26 Thrombotic complications in patients with HIT typically affect large vessels, with VTE occurring more frequently than arterial events.27 Multiple thrombotic events are not uncommon,27 and patients with catastrophic outcomes involving multiple vascular beds have been reported.28,29 Although HIT most often presents with macrovascular thrombosis, severe HIT complicated by DIC can be associated with limb ischemia and microvascular thrombosis, even in the absence of deep vein thrombosis (DVT) or treatment with warfarin.30,31 In addition, patients with acute HIT who are treated with warfarin can develop warfarin-induced skin necrosis32 and/or venous limb gangrene,33 due to microvascular occlusions secondary to acquired deficiency of protein C.
Recently, several reports have described patients with an entity referred to as “spontaneous” HIT.34-38 These patients present with venous and/or arterial thromboembolic events and may be thrombocytopenic at the time of presentation,34,39 or rapidly develop thrombocytopenia once heparin is administered to treat the thrombotic event(s).38,40 Several of these patients presented with multiple events, or with the development of new thromboembolic occlusions over a brief period of time. Antecedent events, have been reported, including surgical procedures (without evident exposure to heparin)36,37 and infections.35 Testing for antibodies to PF4/heparin is positive, and platelet-activating antibodies can be demonstrated by the serotonin release assay.39 Many of these patients have antibodies capable of activating platelets in the absence of added heparin, but platelet activation is suppressed with excess heparin, consistent with a heparin-dependent antibody.39
The primary treatment of patients with HIT is to stop all heparin exposure and start a nonheparin parenteral anticoagulant, such as argatroban or bivalirudin. Even patients with profound thrombocytopenia can be treated with anticoagulants without hemorrhagic manifestations, although achieving a therapeutic level of anticoagulation with activated partial thromboplastin time (aPTT)-adjusted therapies is challenging if concomitant DIC results in baseline (pretreatment) elevation of the aPTT. Plasma exchange and rituximab have been used as adjunctive therapies in patients with refractory HIT with thrombosis.41
Cancer-associated thrombosis
Patients with cancer have an increased risk for VTE42 as well as a greater likelihood to have recurrent thrombotic events despite appropriate therapy.43 Although thromboembolism is a leading cause of death in patients with cancer,44 most of these patients do not present with a catastrophic thrombotic phenotype. Trousseau syndrome, however, can be more aggressive, characterized by migratory thrombotic events in association with an underlying malignancy.45 Thromboembolic events may involve deep or superficial veins or the arterial circulation, and may be associated with nonbacterial thrombotic endocarditis (particularly arterial events) and DIC, which can complicate anticoagulant therapy.46 The primary treatment of patients with cancer-associated thrombosis consists of anticoagulant therapy with LMWH, but patients with multiple, particularly recurrent, events may benefit from an increased dose with consideration given to measure anti–factor Xa levels to tailor therapy.47 Warfarin may be ineffective in patients with severe Trousseau syndrome,48 and has been associated with venous limb gangrene in patients with cancer and thrombosis, similar to patients with HIT.49-51 Rivaroxaban has also been associated with venous limb gangrene in a patient with acute cancer-associated thrombosis.52
Patients with malignancy who develop catastrophic APS have been reported,53 and 9% of patients reported in the CAPS Registry had cancer.54 Patients with malignancy and catastrophic APS should be treated similar to noncancer patients with catastrophic APS, with anticoagulation, steroids, and plasma exchange.54 Cancer patients may also develop a microangiopathic hemolytic anemia which is usually not responsive to plasma exchange or anticoagulant therapy.55
Other disorders
Catastrophic thrombotic events have been described in patients with idiopathic hypereosinophilic syndrome,56,57 Kimura disease,58 inflammatory bowel disease,2,59 and Behçet disease.60,61 Behçet disease is associated with an increased risk for VTE, and, to a lesser extent, arterial occlusions and aneurysms.62 The most common thrombotic complication in patients with Behçet disease is DVT, and patients frequently have recurrent events. Different types of vascular involvement tend to cluster, such as dural sinus thrombosis and pulmonary artery thrombosis.63 Anticoagulant therapy is the primary initial intervention in these patients. Homozygous deficiency of protein C or protein S is associated with neonatal purpura fulminans, which, in some cases, includes large vessel occlusions.64,65 Catastrophic venous thrombosis in a young adult with protein C deficiency has also been reported.66 Management includes replacement of the deficient natural anticoagulant with fresh-frozen plasma or protein C concentrates.
“Idiopathic” catastrophic thromboembolism
A subset of patients presenting with multiple thrombotic events will have none of the above disorders. Thrombotic complications include VTE, but these patients also sustain thrombotic events in unusual locations, including the hepatic veins, portal vein, inferior vena cava (IVC), and cerebral venous sinuses.2,3 The arterial circulation is frequently involved, including myocardial infarction, stroke, transient ischemic attacks, and peripheral arterial embolism. A thrombotic microangiopathy may occur, including purpura fulminans and microvascular occlusions affecting internal organs; bilateral adrenal apoplexy has also been described.1 Multiple thrombotic events may present simultaneously or rapidly progress despite adequate anticoagulation, a clinical course we have referred to as thrombotic storm.1,2
The patient described at the beginning of this article had no known hypercoagulable disorder, and all laboratory testing during the event was negative. Although it is possible that testing simply missed a hypercoagulable state because of his fulminant coagulopathy, he is best described as having “idiopathic” catastrophic thromboembolism.
Diagnostic approach to the patient presenting with catastrophic thrombosis
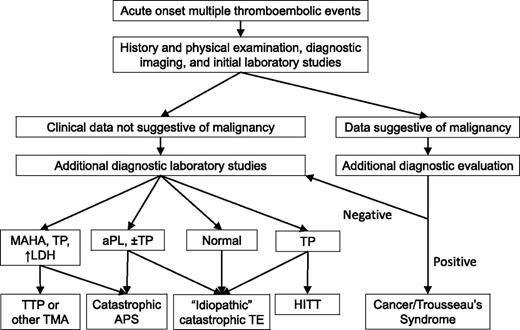
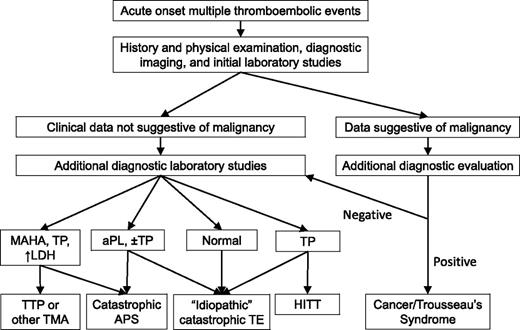
Initial diagnostic evaluation of a patient presenting with multifocal, or rapidly progressive, thrombotic events includes a thorough history and physical examination, relevant imaging studies, and clinical laboratory data (Figure 1). The history needs to cover symptoms associated with the acute thrombotic event(s), including timing, location, pace of progression, response, if any, to initial (or ongoing) therapies, prior thrombotic events, and prior diagnosis of APS or aPL, or TTP. Potential precipitating events should be identified, including recent illnesses, procedures, minor trauma, and medication changes, including recent heparin exposure. For women of reproductive age, use of oral contraceptives or the possibility of pregnancy needs to be considered. For pregnant women, recent changes need to be reviewed (eg, new hypertension, urinary protein levels). Prior personal or family history of thrombosis would suggest an inherited thrombophilia might be present. A thorough review of symptoms helps determine whether one needs to consider searching for an underlying malignancy or rheumatologic disorder.
Diagnostic approach to the patient with a catastrophic thrombotic presentation. The presenting history and physical examination, initial imaging studies, and laboratory studies are used to determine the most likely etiology of the patient’s thrombotic diathesis. Initial laboratory studies should include a complete blood count and blood film, a comprehensive metabolic panel (including blood urea nitrogen, creatinine, and liver function studies), an LDH, a prothrombin time, partial thromboplastin time, fibrinogen, and D-dimer, and testing for aPLs. Additional diagnostic laboratory studies that might be obtained in selected subsets of these patients would include testing for anti-PF4/heparin antibodies (to evaluate for HIT), an ADAMTS13 level (to evaluate for an atypical presentation of TTP), additional testing for aPLs (to evaluate for catastrophic APS), and selected additional tests that may direct or alter therapy (eg, antithrombin level). There can be considerable overlap between these disorders, particularly at initial presentation. aPL, antiphospholipid antibody; APS, antiphospholipid syndrome; HITT, heparin-induced thrombocytopenia with thrombosis; LDH, lactate dehydrogenase; MAHA, microangiopathic hemolytic anemia; TMA, thrombotic microangiopathy; TP, thrombocytopenia; TTP, thrombotic thrombocytopenic purpura.
Diagnostic approach to the patient with a catastrophic thrombotic presentation. The presenting history and physical examination, initial imaging studies, and laboratory studies are used to determine the most likely etiology of the patient’s thrombotic diathesis. Initial laboratory studies should include a complete blood count and blood film, a comprehensive metabolic panel (including blood urea nitrogen, creatinine, and liver function studies), an LDH, a prothrombin time, partial thromboplastin time, fibrinogen, and D-dimer, and testing for aPLs. Additional diagnostic laboratory studies that might be obtained in selected subsets of these patients would include testing for anti-PF4/heparin antibodies (to evaluate for HIT), an ADAMTS13 level (to evaluate for an atypical presentation of TTP), additional testing for aPLs (to evaluate for catastrophic APS), and selected additional tests that may direct or alter therapy (eg, antithrombin level). There can be considerable overlap between these disorders, particularly at initial presentation. aPL, antiphospholipid antibody; APS, antiphospholipid syndrome; HITT, heparin-induced thrombocytopenia with thrombosis; LDH, lactate dehydrogenase; MAHA, microangiopathic hemolytic anemia; TMA, thrombotic microangiopathy; TP, thrombocytopenia; TTP, thrombotic thrombocytopenic purpura.
The physical examination needs to assess the location and extent of thrombotic events, to guide decisions about imaging studies, determine the need for urgent intervention, and provide a baseline examination to assess response to therapy. Identification of lymphadenopathy, palpable masses or organomegaly, jaundice, or other unexpected findings should prompt evaluation for an underlying malignancy.
Imaging studies are needed to confirm the location, distribution, and overall burden of thrombosis. An echocardiogram is useful for identifying cardiac valvular disease, intracardiac thrombosis, interatrial shunts in patients with arterial thromboembolism, and right heart compromise in patients with pulmonary embolism (PE). Imaging for an occult malignancy is not recommended in the absence of clinical symptoms or findings.
The initial laboratory findings are important to confirm the diagnosis and guide management decisions (Figure 1). Thrombocytopenia may be seen with any of these conditions, but the concomitant presence of schistocytes and an elevated lactate dehydrogenase (LDH) would favor a thrombotic microangiopathy. Catastrophic APS can also have laboratory evidence for a microangiopathic process, however, and, in the absence of a prior history of APS or SLE, a patient with catastrophic APS can appear very similar to a patient with TTP.
Isolated thrombocytopenia on presentation may be seen in patients with spontaneous (or delayed) HIT (Figure 1), but these patients may also present with a normal platelet count that drops rapidly with the initiation of heparin therapy. Coagulation studies are normal for some of these patients, but others present with or rapidly develop abnormal studies. A prolonged aPTT may signify the presence of a lupus anticoagulant, which is seen in >80% of patients with catastrophic APS.10 A prolonged prothrombin time (PT) and/or aPTT should prompt evaluation for DIC. Abnormal renal or liver function studies may identify affected organs that can impact the choice of anticoagulant agent.
Several additional laboratory studies can be helpful in the management of these patients (Table 1). An ADAMTS13 level, which is typically low in patients with TTP,16 should be sent prior to initiating plasmapheresis. Positive results for a lupus anticoagulant, moderate-to-high titer anticardiolipin antibodies, and/or moderate-to-high titer anti–β2-glycoprotein I antibodies are suggestive of catastrophic APS. Patients with thrombocytopenia should be evaluated for anti-PF4/heparin antibodies, first by enzyme-linked immunosorbent assay (ELISA), and, if positive, confirmed by a functional assay.39 Decisions concerning anticoagulant therapy must not wait on any of these results, however, because delays in initiating appropriate therapy can be rapidly fatal. These laboratory studies may provide critical information that impacts management decisions after the first 24 to 48 hours, however, and should be obtained depending on clinical concern.
Our patient’s initial laboratory studies were unremarkable, but he subsequently developed DIC with marked hypofibrinogenemia and thrombocytopenia. He did not have notable microangiopathic changes, however.
Therapeutic management of patients presenting with catastrophic thrombosis
Given the lack of clinical trials defining the optimal therapeutic approach for patients with catastrophic thrombotic disorders, the following recommendations are empiric and based on review of available data and the authors’ collective experiences. Prospective studies are needed, but the logistical difficulties of conducting such studies in these rare disorders are significant.
For patients presenting with multiple thrombotic events, several fundamental principles should be followed to provide the best chance for a good outcome. First, therapeutic anticoagulation, without interruption, is the most critical component in the management of these patients. Second, additional therapies, such as plasmapheresis and/or immunomodulatory agents, should be instituted promptly if catastrophic APS or atypical TTP is suspected. Third, ongoing management requires daily oversight and review of the patient’s response to therapy and development of new complications. Fourth, management of these patients frequently requires the collaborative involvement of specialists from several areas. This team must work together to provide a coordinated strategy and manage the diverse complications that may develop, including perioperative treatment, management of concomitant hemorrhagic complications, and integration of multiple therapeutic modalities. An early approach to management based on initial laboratory data is presented in Table 3.
Antithrombotic therapy
The importance of continuous, therapeutic anticoagulation is underscored by the thrombotic complications that developed in patients when anticoagulant therapy was withheld, even briefly, in the report by Kitchens.1 Unfractionated heparin is an effective anticoagulant in this setting, given the ability to titrate dose, familiarity with management in the periprocedural setting, and availability of a reversal agent, which should be reserved for cases of disastrous hemorrhagic complications only. If the baseline aPTT is prolonged, adequacy of heparin anticoagulation should be confirmed with an anti–factor Xa assay (targeting the upper end of the recommended heparin level of 0.3 to 0.7 anti–factor Xa units/mL), given the high frequency of lupus anticoagulants in this population. In addition, fibrinogen and D-dimer levels should be checked if the aPTT (and/or PT) is prolonged, to evaluate for DIC. Depending on the cause of the prolonged aPTT, using the aPTT alone may lead to subtherapeutic levels of anticoagulation and increased risk for recurrent thromboembolism. LMWH could be substituted for heparin, but it needs to be dose-adjusted in patients with renal insufficiency and can be more difficult to manage in the periprocedural setting.
Rapidly achieving target therapeutic anticoagulant levels is critical in these patients. In certain situations, patients may require unusually high doses of IV heparin to achieve a therapeutic aPTT (eg, >25 units/kg per hour), a phenomenon referred to as “heparin resistance.”67 Several potential mechanisms have been identified for heparin resistance, including high levels of factor VIII, fibrinogen, and other heparin-binding acute-phase reactants,68 and decreased levels of antithrombin.69 Measuring anti–factor Xa levels can help with patient management,70 although we recommend interpreting these levels in the context of an aPTT obtained concomitantly. Heparin resistance due to antithrombin deficiency has been reported in patients undergoing cardiac bypass surgery and during extracorporeal membrane oxygenation, and antithrombin supplementation has been used to achieve effective anticoagulation.71 The value of antithrombin supplementation remains unclear, however, and switching from heparin to a parenteral direct thrombin inhibitor is an alternative approach.72 Elevated factor VIII levels can potentially interfere with using the aPTT to monitor argatroban, which should be suspected in patients who appear to exhibit “argatroban resistance.”73
Patients with thrombocytopenia on presentation, or who develop thrombocytopenia shortly after starting heparin, should be considered as potentially having spontaneous HIT (or delayed HIT, if recently exposed to heparin). We recommend checking the anti-PF4/heparin antibody level and simultaneously switching the patient to argatroban or bivalirudin. Alternatively, fondaparinux could be used if renal function is acceptable and no acute interventional procedures are planned. If the anti-PF4/heparin antibody ELISA returns negative, the patient could be switched back to heparin or LMWH. Given the high likelihood of false-positive results with the immunoassays, particularly with low-positive results, we recommend confirming positive immunoassay results with a functional assay (eg, serotonin release assay).
Thrombolytic therapy should be considered for patients who present with massive PE or extensive DVT. Thrombolytic therapy may also be considered in patients who present with acute stroke or peripheral arterial occlusion. Catheter-directed thrombolytic therapy is preferred for certain events, such as extensive DVT, if expertise is available. Mechanical thrombectomy and stent placement may be helpful in certain patients, including those with extensive iliofemoral thrombosis.74,75
Antiplatelet therapy may be used in addition to anticoagulant therapy, particularly for patients with arterial thromboembolic events. However, we would not recommend replacing anticoagulant therapy with antiplatelet therapy in the acute setting. Dual antiplatelet therapy may be desired for patients having a stent placed; we would still recommend incorporating anticoagulation as part of the patient’s regimen, depending on the spectrum of events.
A subset of patients will have progressive thrombotic events despite apparently therapeutic, or even supratherapeutic, levels of anticoagulation. We first confirm the degree of anticoagulation by laboratory testing, and then either increase the dose of the anticoagulant (if not supratherapeutic) or switch to an alternative anticoagulant. Depending on the situation, one could also consider adding an antiplatelet agent or an immunomodulatory agent.
Anticoagulant therapy with heparin was re-initiated once his DIC began to improve. Anti–factor Xa levels were used initially given the abnormal aPTT results. Testing for HIT, prompted by his thrombocytopenia, was negative.
Inferior vena cava filter
An IVC filter may be considered in some patients with catastrophic thrombotic syndromes, particularly if significant hemorrhagic complications develop or in the setting of a massive PE, cardiac compromise, and residual DVT. In general, we strongly recommend avoiding IVC filters in the acute setting, as these devices can be associated with a variety of adverse events, including IVC thrombosis and PE.76 IVC filter placement in patients with HIT can exacerbate thrombotic complications,77 even in the setting of appropriate anticoagulant therapy.78 If an IVC filter is placed, we recommend use of a retrievable filter, concomitant use of anticoagulant therapy, if possible, and filter retrieval as soon as possible.
Plasma exchange
Plasma exchange is frequently used in patients with catastrophic APS, and is the primary treatment of patients with TTP. For patients presenting with a catastrophic thrombotic syndrome, we consider plasma exchange in those who appear to have catastrophic APS and a prominent TTP-like presentation, in addition to anticoagulation. We recommend sending an ADAMTS13 level prior to initiating plasma exchange, to help establish the diagnosis. Decisions concerning how long to continue plasma exchange are based on clinical response to the procedure (eg, normalization of platelet count, absence of schistocytes on the blood film).
Immunomodulatory agents
Immunomodulatory therapies are essential for the successful treatment of patients with catastrophic APS.79 In addition to anticoagulant therapy and plasma exchange, prednisone and IV immunoglobulin are most commonly used. Less frequently, these patients have been treated with cyclophosphamide,79 azathioprine,79 rituximab,80 and eculizumab.15 Immunomodulatory therapies, particularly prednisone and rituximab, are also frequently used for TTP, but typically not for the other catastrophic thrombotic disorders.
Surgical procedures
Surgical thromboembolectomy may be necessary in patients with massive PE, particularly with cardiac compromise, or in patients with acute peripheral arterial occlusions. Amputation of infarcted limbs resulted in the remission of catastrophic APS in 2 cases, presumably related to removal of chronic leg ulcers. These patients are at extremely high risk for thrombosis during interventional and surgical procedures. For required procedures, medical and surgical teams need to collectively devise a plan to minimize thrombotic as well as hemorrhagic risks.81
Catastrophic thrombotic syndromes during pregnancy
Catastrophic APS has been described in patients who were pregnant or in the peripartum period, including patients with known APS during transition from warfarin to LMWH initiated because of pregnancy.82,83 Patients without APS or other disorders can also develop a severe thrombotic syndrome during pregnancy or in the early postpartum setting.2,84 Presentation may overlap with other pregnancy-related syndromes, including HELLP (hemolysis, elevated liver enzymes, low platelet count), preeclampsia, and eclampsia.83
Anticoagulant therapy is essential, using LMWH early in the pregnancy, and unfractionated heparin around the time of delivery. Steroids, plasma exchange, and/or IV immunoglobulins can be used in pregnant patients presenting with catastrophic APS. Early delivery may be necessary in certain situations, such as in the presence of HELLP or eclampsia as well as “idiopathic” catastrophic thrombosis.
Platelet counts need to be followed closely in patients on heparin or LMWH during pregnancy because thrombocytopenia may develop for several reasons, and HIT during pregnancy, although rare, has been reported. Historically, danaparoid was used in these patients, but this agent is no longer available in the United States. Argatroban85 and fondaparinux86 have been used in this clinical setting. We recommend that these patients be managed at thrombosis centers with expertise in hematology and high-risk maternal-fetal medicine.
Long-term management
We generally recommend that patients with catastrophic macrovascular thrombotic events be treated with indefinite anticoagulant therapy. Although a provoking event may have triggered the initial thrombotic event, the outcome is atypical and extreme, and we consequently favor treating these patients with a long-term antithrombotic strategy.
Our patient’s thrombotic diathesis continued to progress during DIC and did not improve when heparin therapy was re-initiated. Abnormal coagulation studies not only raised concerns about potential hemorrhagic risk, but also interfered with the management of his anticoagulant therapy. The extensive pulmonary emboli and DVTs were not identified prior to his death.
Future directions
A better understanding of the catastrophic thrombotic syndromes, including what precipitates them and what drives the extensive, multicentric thrombotic process, is essential. The Thrombotic Storm Study Group was formed in 2009 (http://hihg.med.miami.edu/thromboticstorm), in response to the untimely and unexpected death of the patient presented in this article. The primary objective of this group is to investigate and characterize genetic and acquired risk factors associated with these catastrophic thrombotic disorders, in order to elucidate the basis of the extreme prothrombotic state, and to develop novel strategies to halt thrombotic progression.
Acknowledgments
The authors thank their colleagues in the Thrombotic Storm Study Group, the providers who have contacted us with questions and referrals of these complex patients, and the patients who have participated in research studies to better understand this disorder. The authors welcome any inquiries concerning patients with catastrophic thrombotic syndromes.
Authorship
Contribution: T.L.O., D.E., and C.S.K. all contributed to the writing and review of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas L. Ortel, Division of Hematology, Duke University Medical Center, Box 3422, Stead Building, Durham, NC 27710; e-mail: thomas.ortel@duke.edu.