Key Points
Independent prognostic relevance of quantitative Bcl-2/IgH monitoring in the PB of patients with FL before and after first-line therapy.
Abstract
Bcl-2/IgH rearrangements can be quantified in follicular lymphoma (FL) from peripheral blood (PB) by polymerase chain reaction (PCR). The prognostic value of Bcl-2/IgH levels in FL remains controversial. We therefore prospectively studied PB Bcl-2/IgH levels from 173 first-line FL patients who were consecutively enrolled, randomized, and treated within the multicenter phase 3 clinical trial NHL1-2003 comparing bendamustine-rituximab (B-R) with rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone. From April 2005 to August 2008, 783 pre- and posttreatment PB samples were quantified by quantitative PCR. At inclusion, 114 patients (66%) tested positive and 59 (34%) were negative for Bcl-2/IgH. High pretreatment Bcl-2/IgH levels had an adverse effect on progression-free survival (PFS) compared with intermediate or low levels (high vs intermediate: hazard [HR], 4.28; 95% confidence interval [CI], 1.70-10.77; P = .002; high vs low: HR, 3.02; 95% CI, 1.55-5.86; P = .001). No PFS difference between treatment arms was observed in Bcl-2/IgH-positive patients. A positive posttreatment Bcl-2/IgH status was associated with shorter PFS (8.7 months vs not reached; HR, 3.15; 95% CI, 1.51-6.58; P = .002). By multivariate analysis, the pretreatment Bcl-2/IgH level was the strongest predictor for PFS. Our data suggest that pre- and posttreatment Bcl-2/IgH levels from PB have significant prognostic value for PFS in FL patients receiving first-line immunochemotherapy. This trial was registered at www.clinicaltrials.gov as #NCT00991211 and at the German Federal Institute for Drugs and Medical Devices as #BfArM-4021335.
Introduction
Follicular lymphoma (FL) is the most common subtype of indolent non-Hodgkin lymphoma (NHL). Its clinical course is characterized by relapses, indicating the incurability of FL with currently available therapies.1-3 Minimal residual disease (MRD) assessment may provide significant prognostic value in FL.4-7 The Bcl-2/IgH hybrid gene, resulting from the reciprocal chromosomal translocation t(14;18)(q31;q21) provides an ideal genetic target for molecular MRD assessment and monitoring, given that it is present in up to 85% of FLs.8,9 Quantitative PCR (qPCR) techniques have enabled accurate detection of Bcl-2/IgH levels and kinetics.5-7,10 Moreover, intrapatient quantifications from paired bone marrow (BM) and peripheral blood (PB) samples demonstrated high concordance, suggesting that PB is the most suitable source for serial Bcl-2/IgH assessment in FL.6,11,12
Although it has been demonstrated across various treatment modalities that conversion to Bcl-2/IgH negativity in FL results in prolonged progression-free survival (PFS) and overall survival,13-16 the prognostic value of pre- and posttreatment Bcl-2/IgH levels from either BM or PB are still discussed controversially.4,7,17 Several confounding factors contributing to this controversy include assay sensitivity, sample source, patient population, and evolving treatment modalities. Standard chemotherapy does not achieve disease eradication from the PB or BM in the majority of patients,7,10,14,15 whereas rituximab as single agent or in combination with chemotherapy leads to a complete clearance of Bcl-2/IgH-positive cells in most patients,5,18 indicating that Bcl-2/IgH levels in PB are strongly affected by rituximab use.
We therefore designed the study presented here to prospectively evaluate the prognostic value of serial Bcl-2/IgH quantification from PB in previously untreated FL patients who were randomized and treated within the previously reported NHL1-2003 trial comparing bendamustine plus rituximab (B-R) with rituximab plus cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP).19 Our objectives were to prospectively determine (1) whether pretreatment Bcl-2/IgH levels correlated with disease characteristics, treatment response, and outcome; (2) whether induction of Bcl-2/IgH negativity posttherapy was associated with a favorable outcome; and (3) whether sequential MRD quantification during follow-up predicted the likelihood of clinical relapse.
Patients and methods
Study design and patients
In the multicenter, randomized, open-label NHL1-2003 study, conducted by the German Study group indolent Lymphomas (StiL; www.clinicaltrials.gov, #NCT00991211), patients with newly diagnosed, treatment-naive FL were randomized to receive 6 cycles of either bendamustine (90 mg/m2 days 1 and 2; once per month) plus rituximab (375 mg/m2) or rituximab (375 mg/m2) plus standard CHOP (3 weekly).19 Disease evaluation by computed tomography scan was performed at baseline, after 3 cycles and after the last cycle of therapy. Follow-up examinations including laboratory test, physical examination, and sonography and/or chest X-ray were performed every 3 months. Follow-up computed tomography imaging was performed ad hoc depending on the clinical findings or at least every 6 months.
From April 2005 to August 2008, the last 173 consecutive patients with FL of the NHL1-2003 were included in the MRD study (Figure 1). After giving written informed consent, the PB of all patients was screened for Bcl-2/IgH rearrangements within the MBR by qPCR. As a control, MBR Bcl-2/IgH-negative samples were reanalyzed for both MBR and minor cluster region Bcl-2/IgH-rearrangements by qualitative nested PCR as previously described6 (supplemental Table 1; see the Blood Web site). MBR Bcl-2/IgH-positive patients were selected for sequential MRD follow-up. Samples were taken pre- and posttreatment and every 3 months during follow-up. The MRD study was approved by all local ethics committees of each participating institution.
Consolidated Standards of Reporting Trials diagram. PB samples from the final consecutive 173 patients with newly diagnosed FL, treated within the clinical study NHL1-2003 conducted by the German StiL study group, were analyzed for Bcl-2/IgH rearrangements. A total of 107 patients (62%) had a detectable Bcl-2/IgH rearrangement [(q32;q21)(IgH/bcl2)] within the major breakpoint region (MBR) and were selected for serial Bcl-2/IgH quantification.
Consolidated Standards of Reporting Trials diagram. PB samples from the final consecutive 173 patients with newly diagnosed FL, treated within the clinical study NHL1-2003 conducted by the German StiL study group, were analyzed for Bcl-2/IgH rearrangements. A total of 107 patients (62%) had a detectable Bcl-2/IgH rearrangement [(q32;q21)(IgH/bcl2)] within the major breakpoint region (MBR) and were selected for serial Bcl-2/IgH quantification.
Procedures
PB samples were placed in EDTA tubes and shipped within 24 hours to the reference laboratory at the Department of Hematology at Heinrich-Heine University, Duesseldorf, where qPCR analysis was performed. Following density centrifugation, genomic DNA from 5 × 106 mononuclear cells (MNCs) was extracted using the Qiagen Blood Mini Kit.
A noncommercially available improved version of the LightCycler MBR Quantification Kit, specific for the t(14;18)(q32;q21) (IgH/Bcl-2) translocation, was provided by Roche Diagnostics (Penzberg, Germany). qPCR was performed according to the manufacturer’s instructions. Briefly, 50 µL of sample DNA was amplified on the LightCycler using target-specific primers as well as specific hybridization probes for detection of the amplicon (supplemental Methods). The DNA amounts added to the reaction mixtures ranged between 20 and 100 ng/µL (mean, 90 ng/µL or 4500 ng/reaction; standard deviation, 23 ng/µL). The PCR had a sensitivity of 2 × 10−6 validated in dilution assays (supplemental Figure 1).
The final result (referred to as Bcl-2/IgH level) was calculated by the LightCycler Relative Quantification Software using a calibrator DNA for reference and expressed as a ratio of target (Bcl-2/IgH) to reference gene (tPA) as previously described.6
Stratification of patients according to their pretreatment Bcl-2/IgH levels was based on the scheme of Rambaldi and colleagues: high (Bcl-2/IgH/tPA ratio >1), intermediate (ratio 0.1-1), and low (ratio <0.1).7
Statistical analysis
PFS was calculated from start or end of therapy to the respective event, depending on the time of MRD assessment.20 Relapse, disease progression, or any additional treatment was counted as an event. Survival curves were estimated according to the Kaplan-Meier method,21 the log-rank test was performed for comparisons, and a Cox regression model with either a step-wise backward variable selection approach (P < .1) or enter method was applied for multivariate analysis and to obtain hazard ratio (HR). Differences in Bcl-2/IgH levels between patient groups according to clinical parameters were calculated using the Mann-Whitney U test. To identify prognostic variables, the following factors were examined by univariate and multivariate analysis using a multiple regression model: age, sex, serum levels of lactate dehydrogenase (LDH) and β2-microglobulin, number of involved lymph nodes (LNs), Ann Arbor stage, BM and spleen involvement, pretreatment Bcl-2/IgH level, posttreatment Bcl-2/IgH status, remission categories, Follicular Lymphoma International Prognostic Index (FLIPI),22 and International Prognostic Index scores.23 All statistical analyses were performed using SPSS software.
Results
In total, 783 PB samples representing 173 FL patients from randomization up to 69 months after therapy were analyzed. Clinical follow-up was recorded for all patients. At diagnosis, 114 of 173 patients (66%) had a PCR-detectable Bcl-2/IgH rearrangement. The majority of patients (107, 62%) were positive for the MBR, and 7 patients (4%) were positive for the minor cluster region chromosomal breakpoint. Pretreatment Bcl-2/IgH levels were quantified for all 107 MBR Bcl-2/IgH-positive patients. Posttreatment MRD follow-up was performed on 92 of 107 MBR Bcl-2/IgH-positive patients; baseline clinical characteristics are summarized in supplemental Table 2.
Pretreatment: no relevance of pretreatment Bcl-2/IgH status
The overall response rate and complete response (CR) rates after first-line therapy were 90.7% and 26.7% for 86 patients receiving R-CHOP and 90.8% and 36.8% for 87 patients treated with B-R. Three patients (3.5%) receiving R-CHOP and 6 patients (6.9%) receiving B-R had progressive disease. No differences with regard to sex, age, Ann Arbor stage, bulky disease, B-symptoms, BM involvement, elevated LDH serum level, FLIPI score, and treatment response were found between Bcl-2/IgH-negative and Bcl-2/IgH-positive patient groups.
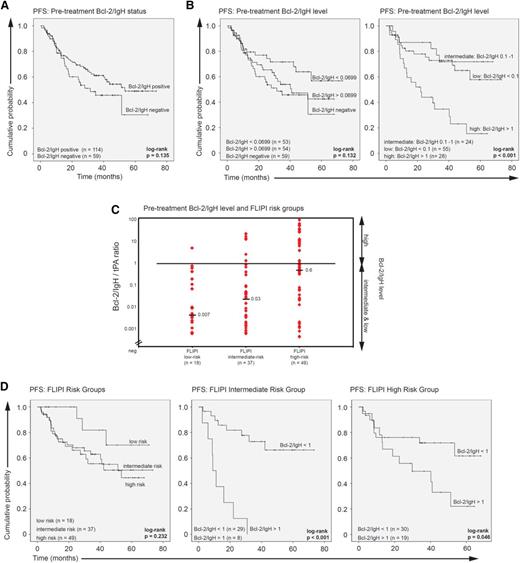
Analysis of the effect of the pretreatment Bcl-2/IgH status on median PFS from first randomization showed no significant difference between positive and negative patients (positive vs negative, 53.4 vs 31.6 months; HR, 0.70; 95% CI, 0.43-1.12; P = .14; Figure 2A).
Pretreatment Bcl-2/IgH status and level. (A) Kaplan-Meier plots of PFS according to the pretreatment Bcl-2/IgH status: Bcl-2/IgH positive vs Bcl-2/IgH negative. (B) Kaplan-Meier plots of PFS according to the pretreatment Bcl-2/IgH level: negative vs below median vs above median (Bcl-2/IgH level of negative vs <0.0699 vs >0.0699) and high vs intermediate vs low (Bcl-2/IgH level of >1 vs 0.1-1 vs <0.1). (C) Pretreatment Bcl-2/IgH level and FLIPI risk groups: low risk (score 0-1), intermediate risk (score 2), and high risk (score >3-5). Horizontal bars indicate the median Bcl-2/IgH level. Diamonds correspond to individual sample values. (D) Kaplan-Meier plots of PFS for FLIPI risk groups low, intermediate, and high. PFS for intermediate- and high-risk patients was further calculated according to the pretreatment Bcl-2/IgH levels.
Pretreatment Bcl-2/IgH status and level. (A) Kaplan-Meier plots of PFS according to the pretreatment Bcl-2/IgH status: Bcl-2/IgH positive vs Bcl-2/IgH negative. (B) Kaplan-Meier plots of PFS according to the pretreatment Bcl-2/IgH level: negative vs below median vs above median (Bcl-2/IgH level of negative vs <0.0699 vs >0.0699) and high vs intermediate vs low (Bcl-2/IgH level of >1 vs 0.1-1 vs <0.1). (C) Pretreatment Bcl-2/IgH level and FLIPI risk groups: low risk (score 0-1), intermediate risk (score 2), and high risk (score >3-5). Horizontal bars indicate the median Bcl-2/IgH level. Diamonds correspond to individual sample values. (D) Kaplan-Meier plots of PFS for FLIPI risk groups low, intermediate, and high. PFS for intermediate- and high-risk patients was further calculated according to the pretreatment Bcl-2/IgH levels.
Pretreatment: relevance of Bcl-2/IgH levels
In Bcl-2/IgH MBR–positive patients, pretreatment Bcl-2/IgH levels ranged between 0.000281 and 81 with a median value of 0.0699, revealing high interindividual variability among newly diagnosed FL patients. Significantly higher Bcl-2/IgH levels were found in patients with BM involvement (P = .007), spleen enlargement (P = .015), and Ann Arbor stage IV (P = .038). Pretreatment Bcl-2/IgH levels were also proportionally correlated to the number of LNs with disease involvement (>3 LNs, P = .004; >5 LNs, P = .001; >7 LNs, P = .033) and to β2-microglobulin serum levels above 3 mg/L (P = .026). Interestingly, the highest median Bcl-2/IgH levels were detected in 24 patients with elevated β2-microglobulin serum levels (Table 1).
Analysis of the effect of pretreatment Bcl-2/IgH levels on PFS revealed that high Bcl-2/IgH levels (ratio >1, n = 28) adversely impacted PFS. Median PFS was 22.1 months (95% confidence interval [CI], 11.4-32.9 months) in patients with high, not reached in patients with intermediate (ratio 0.1-1, n = 24; high vs intermediate: HR, 4.28; 95% CI, 1.70-10.77; P = .002), and not reached in patients with low pretreatment Bcl-2/IgH levels (ratio <0.1, n = 55; high vs low: HR, 3.02; 95% CI, 1.55-5.86; P = .001; Figure 2B). Comparison of pretreatment Bcl-2/IgH levels between FLIPI risk groups (low risk [score 0-1, n = 18], intermediate risk [score 2, n = 37], and high risk [score 3-5, n = 49]) showed that high Bcl-2/IgH levels were only found in a significant number of FLIPI intermediate- and high-risk patients (Figure 2C). The negative prognostic effect of high pretreatment Bcl-2/IgH levels on PFS was significant in both FLIPI risk groups (FLIPI intermediate risk: Bcl-2/IgH >1 vs <1: 8.9 months vs not reached; HR, 9.27; 95% CI, 3.11-27.65; P < .001; FLIPI high risk: Bcl-2/IgH >1 vs <1: 29.8 months vs not reached; HR, 2.37; 95% CI, 0.99-5.65; P = .05; Figure 2D). Univariate and multivariate analysis identified the pretreatment Bcl-2/IgH level as an independent predictor of poor PFS (Table 2). Collectively, these data demonstrate that pretreatment Bcl-2/IgH quantification in FL identifies a subgroup of FLIPI intermediate- and high-risk patients with a significantly shorter PFS outcome.
Posttreatment: relevance of Bcl-2/IgH status and levels
Next, we analyzed the effect of first-line therapy on posttreatment Bcl-2/IgH status and Bcl-2/IgH levels in 92 patients with available follow-up samples (supplemental Table 2). The overall response rate and CR rates after first-line immunochemotherapy in Bcl-2/IgH positive patients with either B-R or R-CHOP were 94% (86 of 92 patients) and 36% (33 of 92 patients). Fifty-three patients (58%) achieved a partial response (PR), 3 (3%) had stable disease, and 3 (3%) had progressive disease. Three-year PFS was 63%.
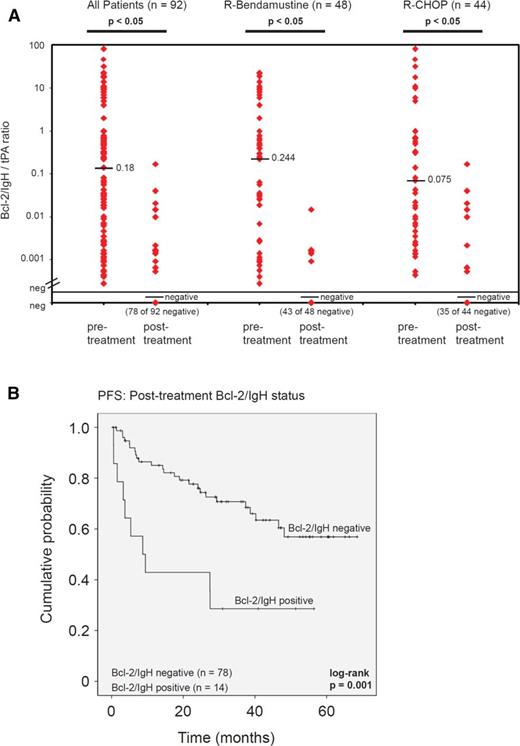
A significant reduction of Bcl-2/IgH levels was observed after 6 cycles of immunochemotherapy: median Bcl-2/IgH level of 0.18 (range 0.000281- 81) vs negative (range negative to 0.17; P = .0005). Conversion from Bcl-2/IgH-positive to Bcl-2/IgH-negative was observed in 78 of 92 patients (85%). Fourteen patients (15%) remained positive but showed a 2 log (median) reduction of their Bcl-2/IgH level after therapy (from 0.660 to 0.00193). Interestingly, the median pretreatment Bcl-2/IgH level in this patient cohort was higher (0.660) when compared with the group of molecular responders who converted (0.049; P = .16). No difference in Bcl-2/IgH response was observed between patients in CR or PR, or between treatment arms at the end of therapy (Figure 3A).
Posttreatment Bcl-2/IgH status and level. (A) Pre- and posttreatment Bcl-2/IgH quantification comparing treatment arms. Horizontal bars indicate the median Bcl-2/IgH level. Diamonds correspond to individual sample values. (B) PFS according to the posttreatment Bcl-2/IgH status.
Posttreatment Bcl-2/IgH status and level. (A) Pre- and posttreatment Bcl-2/IgH quantification comparing treatment arms. Horizontal bars indicate the median Bcl-2/IgH level. Diamonds correspond to individual sample values. (B) PFS according to the posttreatment Bcl-2/IgH status.
In both treatment arms, the posttreatment Bcl-2/IgH status had a significant effect on PFS. Failure to achieve Bcl-2/IgH negativity adversely impacted median PFS (negative vs positive: not reached vs 8.7 months [95% CI, 1.1-16.2 months]; HR, 3.15; 95% CI, 1.51-6.58; P = .002; Figure 3B). The prognostic value of the posttreatment Bcl-2/IgH status remained significant when only patients who achieved CR or PR were analyzed (negative vs positive: not reached vs 27.4 months [95% CI, 7.1-47.7 months]; HR, 2.48; 95% CI, 1.06-5.78; P = .036). In contrast, CR did not significantly improve median PFS when compared with no CR (CR vs no CR: not reached vs 48.2 months; HR, 1.33; 95% CI, 0.65-2.71; P = .44).
Finally, we compared the prognostic relevance of pre- and posttreatment Bcl-2/IgH assessments along with clinical factors. By univariate analysis, both the pretreatment Bcl-2/IgH level and the posttreatment Bcl-2/IgH status were significant predictors for PFS. The FLIPI score, Ann Arbor stages, and the achievement of a CR had no significant impact on PFS. By multivariate analysis, the pretreatment Bcl-2/IgH level was the strongest prognostic factor (P = .0001; Table 3).
Follow-up
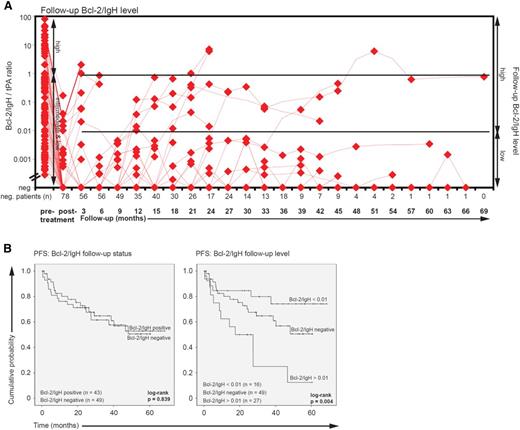
A total of 610 follow-up samples representing 92 patients from 0 to 69 months (median 41) after therapy were analyzed. A posttreatment sample was available for all 92 patients. Of 92 patients, 20 stayed on schedule and had regular 3-monthly BCL-2/IgH follow-up samples taken, whereas 72 patients missed samples (median number 2; range 0-15 missed samples).
Throughout follow-up, 49 patients (53%) remained Bcl-2/IgH negative, and 43 patients (47%) converted to Bcl-2/IgH positivity (Figure 4A). Interestingly, relapse probability was not correlated with Bcl-2/IgH conversion (mean PFS positive vs negative: 44 vs 41 months; HR, 1.07; 95% CI, 0.55-2.08; P = .839), but analysis of the effect of follow-up Bcl-2/IgH levels on PFS revealed that Bcl-2/IgH levels >0.01 (n = 16) adversely impacted PFS (Bcl-2/IgH >0.01 vs <0.01: 17.5 months [95% CI, 0.3-34.7 months] vs not reached; HR, 4.64; 95% Cl 1.66-12.94; P = .003; Figure 4B). The median time to Bcl-2/IgH recurrence was 12 months (range 3 to 63 months), and the interval from Bcl-2/IgH redetection to clinical diagnosis of relapse was 9.5 months (range 0.6 to 20.3 months). These data suggest that patients with a higher relapse probability were identified, but the timing of clinical relapse was not accurately predictable by qPCR.
Follow-up Bcl-2/IgH status and level. (A) Bcl-2/IgH follow-up level. Horizontal bars indicate the median Bcl-2/IgH level. Diamonds correspond to individual sample values. (B) Kaplan-Meier plots of PFS according to the follow-up Bcl-2/IgH status (positive vs negative) and level: high vs low vs negative (Bcl-2/IgH level of >0.01 vs <0.01 vs negative).
Follow-up Bcl-2/IgH status and level. (A) Bcl-2/IgH follow-up level. Horizontal bars indicate the median Bcl-2/IgH level. Diamonds correspond to individual sample values. (B) Kaplan-Meier plots of PFS according to the follow-up Bcl-2/IgH status (positive vs negative) and level: high vs low vs negative (Bcl-2/IgH level of >0.01 vs <0.01 vs negative).
Discussion
Data from this prospective study demonstrate that pre- and posttreatment Bcl-2/IgH levels are both independent prognostic markers for PFS in FL undergoing first-line immunochemotherapy, irrespective of the cytotoxic regimen used in combination with rituximab. Our results also indicate that a Bcl-2/IgH status conversion during follow-up, or in other words a molecular relapse, is not indicative of an inferior PFS outcome unless high Bcl2/IgH levels can be detected.
The enormous interpatient variability of pretreatment Bcl-2/IgH levels observed in this study is in line with previous reports, but the underlying pathophysiological mechanisms are still poorly understood. Consistent with previous reports, we observed significantly higher Bcl-2/IgH levels in patients with BM or spleen involvement and in Ann Arbor stage IV,6,12 suggesting that Bcl-2/IgH levels may simply serve as a surrogate for tumor burden. However, we also show that high Bcl-2/IgH levels had a negative impact on the prognosis of FLIPI intermediate-risk patients, which may suggest that pretreatment Bcl-2/IgH levels also reflect lymphoma cell migration and invasiveness in addition to tumor burden and that enhanced migration in FL may be associated with a poor prognosis and adverse PFS. For instance, of the 28 patients with high pretreatment Bcl-2/IgH levels, 11 (39%) had <7 LNs with disease involvement (median 4, range 1-6), 4 (14%) had no BM and 11 (39%) had no spleen involvement, 21 (75%) had normal LDH serum levels, and only 10 (36%) had elevated β2-microglobulin serum levels.
The major finding of this prospective study is that pretreatment Bcl-2/IgH levels from PB have independent prognostic value for PFS in treatment naive FL. Considering the incurability of the disease, PFS is still considered as the most relevant clinical end point in FL trials. In alignment with our data, Ghielmini and coworkers found that the pretreatment Bcl-2/IgH levels from PB were predictive for response duration to rituximab monotherapy.18 In contrast, other groups reported that the pretreatment Bcl-2/IgH levels from PB, unlike BM, did not allow prediction of PFS.7,10 Possible explanations for these contrasting observations may be differences with regard to patient population studied (first-line vs second-line), treatment modalities (rituximab vs chemotherapy vs rituximab followed by chemotherapy vs the inverse sequence vs combination of both), as well as the qPCR assays used. Interestingly, a high correlation between Bcl-2/IgH levels obtained from paired PB and BM samples was observed in all trials, but PB levels were found to be in general at least 1 log lower compared with the levels detected in BM. This inconsistency rather suggests an assay-dependent limitation of sensitivity than a higher prognostic significance of BM infiltrating lymphoma cells.6,11,12 It seems more likely that accurate prediction of PFS from PB requires an assay with high sensitivity. Consequently, pretreatment Bcl-2/IgH quantification from PB can only be recommended when a sensitive qPCR assay is used (eg, >10−5; supplemental Figure 1).
The molecular remission rate in the PB observed in this trial was 85% (90% R-B vs 80% R-CHOP), which is similar to 80%, 84% and 87% observed in other trials using R-CHOP, rituximab plus mitoxantrone, chlorambucil, prednisolone (R-MCP), or R-fludarabine.5,24,25 The molecular remission rates after rituximab-chemotherapy combinations are markedly higher than those observed after standard chemotherapy or rituximab monotherapy.13,15,16,18 These improvements may directly reflect the benefit of rituximab translating into prolonged PFS.26 Supporting this hypothesis the prognostic value of the posttreatment Bcl-2/IgH status seems accentuated after both rituximab-containing and myeloablative regimens.14,27 According to our data failure to achieve molecular remission after rituximab-chemotherapy has a strong impact on PFS (HR, 3.15; 95% CI, 1.51-6.58; P = .002) and >70% of the patients failing to achieve this goal subsequently relapsed, irrespective of their clinical remission status. Posttreatment Bcl-2/IgH assessment may therefore be specifically useful to identify patients with a poor prognosis who require treatment intensification. Because quantification of posttreatment Bcl-2/IgH levels did not add any further prognostic value in our study, we can only recommend a qualitative posttreatment assessment at present. During follow-up, serial Bcl-2/IgH quantification showed a significant correlation between Bcl-2/IgH levels and PFS. Interestingly, only high Bcl-2/IgH levels during follow-up adversely affected PFS whereas smoldering low Bcl-2/IgH levels irrespective of a detected conversion from Bcl-2/IgH negativity to positivity had no impact on PFS when compared with negative Bcl-2/IgH follow-up levels. These data clearly suggest that long-term disease control is possible despite persistence of the malignant clone. Prolonged tumor dormancy and long-term remissions with persistently detectable Bcl-2/IgH rearrangements in FL have already been reported after radiotherapy28 and conventional chemotherapy,29 suggesting that even combined rituximab-chemotherapy is unable to eradicate the disease. Although our results are concordant with previous reports,7,13-15 the prognostic value of follow-up monitoring is limited because of the indolent course of the disease. This raises the question: what is the value of sequential quantitative Bcl-2/IgH follow-up in FL? A potential value might emerge from the yet unanswered question of the optimal duration of rituximab maintenance therapy in FL patients achieving PR or CR after rituximab-chemotherapy. Clinical trials including the StiL NHL7-2008 are currently underway to define the prognostic value of Bcl-2/IgH monitoring in the context of rituximab maintenance.
In conclusion, our study demonstrates that Bcl-2/IgH quantification before and after therapy allows prognostic evaluation of FL undergoing first-line rituximab-chemotherapy.30 Moreover, these results also suggest that both a positive posttreatment Bcl-2/IgH status as well as high Bcl-2/IgH follow-up levels could be useful to identify patients with poor prognosis that might benefit from intensified or prolonged therapy. However, any predictive value of the qPCR in FL to guide treatment has to be established in prospective trials first.
Presented in part at the 51st annual meeting of the American Society of Hematology, New Orleans, LA, December 7, 2009; and the Annual Meeting of the German, Austrian and Swiss Societies of Hematology and Oncology, Berlin, October 3, 2010.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the teams of the MRD laboratory in Düsseldorf and the StiL study group office, including Anke Boeckmann, Monika Pooten, Annemarie Koch, Hildegard Gausmann, Elke Rosenbaum-König, Irmgard Hamann, Caroline Zörb, Elke Metzler, Helene Weikum, Yvonne Holderer, and Ingeborg Dietz.
This work was supported by funding from Roche Pharma AG, Germany.
Authorship
Contribution: G.K., R.K., and M.J.R. designed the study; F.Z., I.B., T.S., R.F., A.C., G.M., D.K.-K., N.N., G.H., C.L., M.W., W.B., U.G., R.K., J.B., M.J.R., R.H., and G.K. analyzed patients and data; S.P., F.Z., and R.K. performed PCR analyses; F.Z., S.P., and G.K. performed data analyses; F.Z., G.K., R.H., and R.K. wrote the manuscript; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: F.Z. is employed in a for-profit health care company. S.P. received research funding and honoraria. T.S. received honoraria and travel support. A.C. is employed in a for-profit health care company. G.M. received honoraria, had a consulting/advisory role, and received travel support. W.B. received honoraria, F.Z. is employed in a for-profit health care company, Takeda. I.B. has a consultant role for a for-profit healthcare company, Bayer. S.P. received research funding and honoraria by Roche. T.S. received honoraria and travel support by Roche. A.C. is employed in a for-profit health care company, Merrimack Pharmaceuticals. G.M. received travel support by Roche. W.B. received honoraria, had a consulting/advisory role and participated in a speakers' bureau with Roche. U.G. received travel support by Roche. R.K. is employed in a for-profit health care company, Sividon, has been compensated for a leadership role with Sividon, and has a stock or other ownership as well as a patent or other intellectual property with Sividon. J.B. received lecture honoraria and travel support by Roche. M.J.R. received honoraria and had a consulting/advisory role with Roche. G.K. received research funding, honoraria for seminar presentations, and conference travel support by Roche. The remaining authors declare no competing financial interests.
Correspondence: Guido Kobbe, University Hospital Düsseldorf, Heinrich-Heine-University, Medical Faculty, Moorenstrasse 5, 40225 Düsseldorf, Germany; e-mail: kobbe@med.uni-duesseldorf.de.

![Figure 1. Consolidated Standards of Reporting Trials diagram. PB samples from the final consecutive 173 patients with newly diagnosed FL, treated within the clinical study NHL1-2003 conducted by the German StiL study group, were analyzed for Bcl-2/IgH rearrangements. A total of 107 patients (62%) had a detectable Bcl-2/IgH rearrangement [(q32;q21)(IgH/bcl2)] within the major breakpoint region (MBR) and were selected for serial Bcl-2/IgH quantification.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/12/10.1182_blood-2015-03-630012/4/m_1407f1.jpeg?Expires=1769298945&Signature=xGaKG1S3F3dowla5u02K6XLx~wcS-PIcmTQzmVescEPf-LCo1Jui~oY-dGJOhD9AbZ~qWBTo7F02UXZfbBJLEHpxQJW-XAVdBRxLT8XBHe9pps8IT7bjBJEpvI7dbWdF-X4ctS6TMTJF1mCfe06Q9mD7~AGZbh6NR2-yDSbWXg8HsLmnLauAHnnlx2iYq8XfIJV3XfBbOgj5XtUmSBlsqjx0gIrIGj5DIf7boDNUWdKc4EzFeDR~JybOyLtMybXZXagA0Ax-yii72zgWn9X76n-uIJRfB6b7PCZd8DMwduVlBEGqEj8jy9ZqEKuPl-5W2YONZ81a7ALu38JxUToQCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)