Abstract
Background: Therapy induced osteonecrosis has become a limiting toxicity in the intensification of treatment for pediatric acute lymphoblastic leukemia (ALL), particularly among patients 10 to 20 years of age. Prior studies on the genetic determinants of osteonecrosis have focused primarily on patients older than 10 years, leaving the genetic risk factors for the larger group of children with ALL less than 10 years old incompletely understood. It is hypothesized that genetic risk factors may account for a greater proportion of risk of osteonecrosis or involve differing mechanisms in younger than in older patients.
Methods: We performed the first evaluation of genetic risk factors for osteonecrosis in children less than 10 years old using a discovery cohort of 82 cases of osteonecrosis and 287 controls treated on Children's Oncology Group (COG) protocol AALL0331 (NCI standard risk ALL) and tested for replication in 817 children less than 10 treated on COG protocol AALL0232 (high risk ALL). Genotyping was performed using the Affymetrix Gene Chip Human Mapping Array 6.0 and the Illumina Human Exome BeadChip v1.1. A subset of 15 cases in the discovery cohort had coding variant calls verified by whole exome sequencing. Both discovery and replication genome-wide association studies (GWAS) adjusted for demographic and therapy variables known to modify the risk of osteonecrosis. Enhancer enrichment analysis using HaploReg identified tissues affected by the identified single nucleotide polymorphisms (SNPs). Genes associated with the identified SNPs were evaluated using Ingenuity Pathway Analysis for enrichment in biologically relevant pathways.
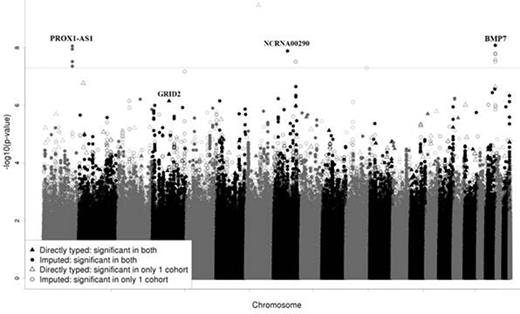
Results: Within the discovery cohort, top ranked variants were rs76599360 and rs77556622 which were in full linkage disequilibrium [P=1.13x10-9, odds ratio (OR) 22.0, 95% confidence interval (95%CI) 8.15-59.6] located near bone morphogenic protein 7 (BMP7). The top replicated SNPs were located near BMP7 [rs75161997, P=5.34x10-8 (OR 15.0; 95%CI 5.64-39.7) and P =0.0498 (OR 8.44; 95%CI 1.002-71.1) in the discovery and replication cohorts, respectively] and PROX1-antisense RNA1 [PROX1-AS1:rs1891059, P=2.28x10-7 (OR 6.48; 95%CI 3.19-13.1) and P=0.0077 (OR 3.78; 95%CI 1.42-10.1) for the discovery and replication cohorts, respectively]. The top replicated non-synonymous SNP, rs34144324, was in a glutamate receptor gene [GRID2, P=8.65x10-6 (OR 3.46; 95%CI 2.00-5.98) and 0.0136 (OR 10.8; 95%CI 1.63-71.4) in the discovery and replication cohorts, respectively], and the genotyping of this variant was verified in the whole exome sequencing data. In a meta-analysis of both cohorts, the replicated BMP7 and PROX1-AS1 variants (rs75161997 and rs1891059, respectively) and a variant in NCRNA00251 (rs141059755) met the genome-wide significance threshold of <5x10-8 (Figure 1). In a meta-analysis of both cohorts, replicated SNPs with meta-analysis P<1 x10-5 showed enrichment in enhancers active in mesenchymal stem cells. Pathway analysis of genes linked to top SNPs (meta-analysis P <0.001) demonstrated enrichment in glutamate receptor signaling and adipogenesis pathways.
Conclusions: Variants in genes important to bone and fat differentiation from mesenchymal stem cells were associated with osteonecrosis in children less than 10 years old. The importance of variants in glutamate receptor signaling in children less than 10 also confirms the findings of a recently completed GWAS of osteonecrosis in AALL0232 (Blood 2014 124:367; 2014) including patients of all ages and in which osteonecrosis occurred primarily in older children. These data provide new insights into osteonecrosis with implications for patients of all ages.
Manhattan plot of meta-analysis for osteonecrosis risk in children <10 years old
Manhattan plot of meta-analysis for osteonecrosis risk in children <10 years old
Hunger:Sigma Tau: Consultancy; Jazz Pharmaceuticals: Consultancy; Merck: Equity Ownership; Spectrum Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract