Abstract
Introduction Precise targeting of Busulfan dose has resulted in improved transplant outcomes following myeloablative conditioning with Fludarabine plus Busulfan (Flu-Bu). However, the impact of Fludarabine pharmacokinetics on outcomes in allogeneic stem cell transplantation (SCT) following myeloablative conditioning remains undefined.
Methods A retrospective single-centre analysis was performed to evaluate the clinical outcomes of 88 patients who received Flu-Bu based conditioning. Patients received Flu (50mg/m2 days-6 to -2), Bu (3.2mg/kg days-5 to -2), and Total Body Irradiation (400 cGy in two doses on day-1). Busulfan dosing was adjusted to achieve a total exposure of 3750 μmol·min/L. Levels of the Fludarabine metabolite 9βD-arabinofuranosyl-2-fluoroadenosine were determined by high performance liquid chromatography-tandem mass spectrometry. Fludarabine exposure, expressed as Fludarabine area under the concentration-time curve (AUC), was calculated on patient blood samples collected on day-5.
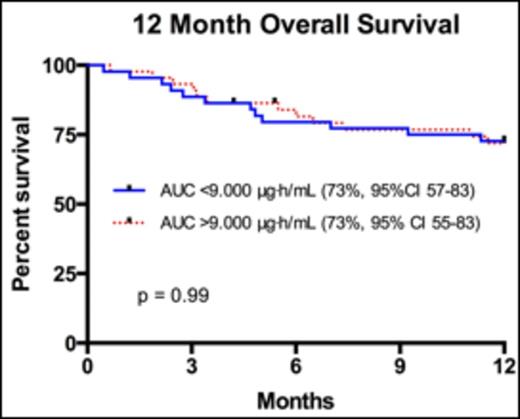
Results Median age of the 88 patients was 49 (range 18-65) years, and median creatinine clearance was 120.5mL/min (range 48-305mL/min). The most common transplanted hematologic malignancies included AML (31%), ALL (20%), and MDS (10%). 32 (36%) received their transplants from HLA-compatible siblings, and 56 (64%) from unrelated donors. Median plasma AUC was 9.038μg·h/mL (range 2.429 - 66.655μg·h/mL). When comparing patients with lower Flu exposure <9.000μg·h/mL versus those with higher Flu exposure >9.000μg·h/mL, there was no significant difference in grade II-IV acute Graft versus Host Disease (aGvHD; 25% versus 32%, p=0.27) or grade III-IV aGvHD (11% versus 14%, p=0.52). Furthermore, there was no difference in average days to engraftment (13.8 versus 13.9, 95% CI 13.01 - 14.72, p=0.77), or average number of infections in 1-year post transplant (2.3 versus 2.5, 95% CI 1.68 - 3.30, p=0.68). Transplant related mortality (TRM) at 100 days was not improved (7.0% versus 11.4%, p= 0.52) with AUC <9.000μg·h/mL. Progression free survival at 3 years with lower Flu exposure <9.000μg·h/mL was 68% (95%CI 53 - 79) compared to higher Flu exposure >9.000μg·h/mL at 59% (95% CI 45-73, p=0.70). Finally, there was no difference in overall survival (OS) at 12 months (Figure 1.0) in patients with an AUC <9.000μg·h/mL (73%, 95% CI 57-83) versus those with AUC >9.000μg·h/mL (73%, 95% CI 55-83, p-value 0.99).
Conclusion In the setting of normal renal function, we have demonstrated that Fludarabine pharmacokinetics does not effect clinical outcomes in myeloablative allogeneic SCT. These data support no role for therapeutic dose monitoring and dose adjustment with Fludarabine in myeloablative conditioning regiments.
Overall survival for patients 12 months after myeloablative SCT with Fludarabine AUC <9.000μg·h/mL compared to patients with Fludarabine AUC >9.000μg·h/mL.
Overall survival for patients 12 months after myeloablative SCT with Fludarabine AUC <9.000μg·h/mL compared to patients with Fludarabine AUC >9.000μg·h/mL.
Off Label Use: Fludarabine in the use of myeloablative conditioning for allogeneic stem cell transplantation..
Author notes
Asterisk with author names denotes non-ASH members.