Abstract
Patients with active chronic graft-versus-host disease (cGVHD) have poor reconstitution of CD4+ CD25+ FOXP3+ regulatory T cells (Tregs), which have broad suppressive activity and are required for the maintenance of peripheral tolerance after allogeneic hematopoietic stem cell transplant. Interleukin-2 (IL-2) is a key growth factor for the development, expansion and function of Tregs in vivo. Phase 1 (DFCI 07-083) and phase 2 (DFCI 11-149) studies of daily subcutaneous low-dose IL-2 in patients with refractory cGVHD demonstrated preferential Treg expansion in all patients and objective clinical responses in approximately 50% of participants. In the phase 2 study, 23 participants with clinical benefit (PR or SD with minor response) continued on extended IL-2 therapy, with 6 and 8 patients receiving over 1 and 2 years of low-dose IL-2, respectively. Analysis of phase 1 study patients indicated that IL-2 restored Treg homeostasis through rapid induction of Treg proliferation, increase in thymic Treg neogenesis and increased Treg expression of the anti-apoptotic Bcl-2 protein. Here, we provide novel insights into the immune homeostatic impact of low-dose IL-2 in phase 2 study patients during the initial 12 week treatment period and during extended therapy.
Proliferation within the Treg compartment, measured by Ki67 expression, rapidly increased and peaked within 1 week of IL-2 initiation. Memory Tregs contained a higher fraction of proliferating cells and correspondingly, a rise in central memory (CM) and effector memory (EM) Tregs preceded an increase in naïve Tregs during the initial 12 week treatment period (Figure 1A and 1B). This is consistent with the observation that memory Tregs are more responsive than naive Tregs to activation by IL-2 in vitro. Although CM and EM Tregs continue to represent the predominant subsets of Tregs, there is a brisk expansion of the naïve Treg subset that coincides with increased thymic output of Tregs during extended IL-2 therapy.
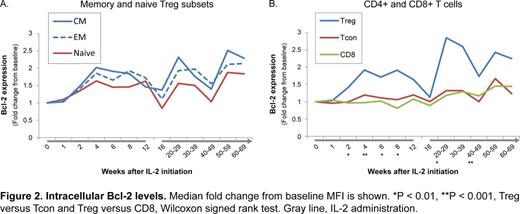
Consistent with their greater proliferative potential, CM and EM Tregs expressed lower levels of Bcl-2 compared with naïve Tregs. However, IL-2 promoted higher Bcl-2 expression in all Treg subsets to similar magnitudes during both the initial 12 week treatment period and extended therapy (Figure 2A). Bcl-2 was preferentially increased in Tregs and not in conventional CD4 (Tcon) or CD8 T cells (Figure 2B). As expected, naïve Tregs expressed very low levels of the pro-apoptotic CD95/Fas receptor protein. CD95 expression in CM and EM Tregs peaked during the period of rapid proliferation within the first week of IL-2 therapy and declined as Bcl-2 expression increased. Changes in CD95 expression levels were similar across all T cell populations. Thus, IL-2 leads to preferential expansion and survival of Tregs in cGVHD patients throughout the duration of therapy.
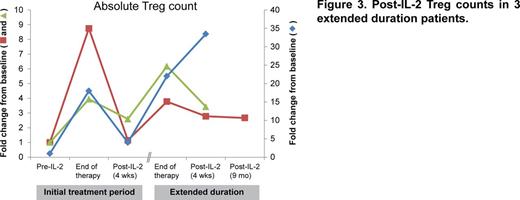
Once IL-2 was discontinued following the initial 12 week treatment period, Treg numbers decreased to pre-treatment baseline levels within 4 weeks, indicating that continuous IL-2 exposure is required for maintenance of enhanced Treg homeostasis. Although patients in the extended duration cohort sustain stably elevated Treg numbers, it is not known whether a long-lasting Treg response is preserved in the absence of exogenous IL-2. Post-IL-2 Treg monitoring results were available for 3 patients in the extended duration cohort. One patient received continuous IL-2 for approximately 3 years and 2 patients stopped after over 1 year of therapy. At 4 weeks after IL-2 discontinuation, 2 of the 3 patients maintained elevated Treg numbers. One patient who stopped IL-2 after 74 weeks due to renal insufficiency had stably elevated Treg numbers and no worsening of cGVHD at 9 months post-IL-2, indicating that some patients may have durable restoration of Treg homeostasis following extended IL-2 therapy (Figure 3).
There were no significant differences in absolute numbers of Treg, Tcon or CD8 subsets between clinical responders and non-responders during the initial 12 week treatment period. Plasma IL-2 and soluble IL-2 receptor levels were also similar between the two groups. Thus, differences in clinical response to IL-2 are likely determined by qualitative differences in Treg or effector T cell function. Functional Treg suppression assays and gene expression profiling studies are in progress to explore this possibility.
Armand:Bristol-Myers Squibb: Research Funding; Merck: Consultancy, Research Funding; Infinity Pharmaceuticals: Consultancy. Soiffer:Gentium SpA/Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Antin:Gentium S.p.A.: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.