Abstract
Introduction
Hox gene expression is high in hematopoietic stem/progenitor cells (HSPCs), decreases during normal differentiation but remains elevated in leukemia subtypes. Polycomb repressor complexes and histone modifiers, e.g. Mixed Lineage Leukemia (MLL), are key regulators of Hox expression. MLL rearrangements, frequent in acute leukemia, are associated high HOXA expression. However, necessity for the HoxA cluster in MLL-leukemia maintenance is not fully elucidated.
Methodology
Ectopic overexpression of MLL-AF9 (MA9) in HSPCs in conditional compound transgenic mouse backgrounds MxCre+/HoxAflox/flox (MAFF) or HoxAflox/flox (AFlox) models resulted in increased colony formation and growth in liquid culture. Transformed colonies, serially re-plated (n=5) in methylcellulose and transplanted into sub-lethally irradiated recipient mice, resulted in primary leukemia. Initially, MAFF-MA9 leukemias were used to examine in vivo deletion of the HoxA cluster using intraperitoneal injections of Poly(I:C) to initiate an interferon response. To further examine the necessity for the HoxA cluster in disease maintenance, AFlox-MA9 leukemias were treated ex vivo with Cre-recombinase (MSCV-Cre-GFP) or vector control (MSCV-GFP), sorted based on GFP expression and used for gDNA-PCR, gene expression (Illumina BeadArray) and transplantation into sub-lethally irradiated recipient mice (500 cGy).
Results
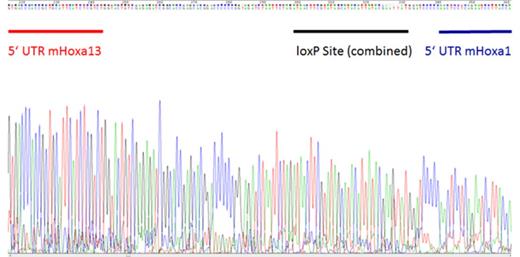
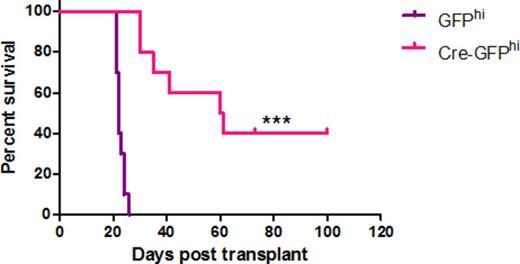
Generation of MLL-AF9 leukemias in the MAFF background (MAFF-MA9) resulted in deletion of one HoxA cluster allele (HoxA+/-), validated by genomic PCR and gDNA sequencing from expanded single colonies (Figure 1) presumably due to viral-induced activation of the Mx1 promoter. PolyI:C treatment of these mice resulted in a modest extension in survival (1-2 days) compared to controls. Luciferase labelling and transplantation of MAFF-MA9 leukemias into NSG mice, followed by PolyI:C treatment, showed a measurable decrease in disease burden compared to control, however this did not correlate with overall survival. Direct treatment of MAFF-MA9 cells with interferon-α (in vitro) resulted in further deletion of the HoxA cluster (HoxA-/hypo) and significant reduction in colony formation compared to controls. Although non-leukemic MAFF HSPCs retained colony forming ability after complete HoxA cluster deletion (HoxA-/-) no HoxA-/- colonies were recovered from the interferon-α treated MAFF-MA9 cultures. Cre-recombinase-induced deletion of the Hoxa cluster from AFlox-MA9 leukemia cells was confirmed by gDNA-PCR and sequencing (Figure 1). Transplantation of Cre-treated AFlox-MA9 cells resulted in significant increased survival (P<0.002) by up to 74 days in recipient mice, compared to controls (Figure 2). Further examination of the leukemias that developed from these Cre-treated AFlox-MA9 cells demonstrated retention of one allele of the HoxA cluster, as a result of escapees. To gain insight into the molecular mechanisms underlying the HoxA requirement for MLL-AF9 maintenance, matched Cre- or control treated AFlox-MA9 samples used for the transplantation were further examined for differential gene expression by Illumina BeadArray analysis. Preliminary analysis of the data has confirmed significant reduction in the expression of HoxA cluster genes (a2, a4, a5, a7, a9) and increased expression of several genes involved in adhesion, differentiation or immune response (e.g. Itgb3bp, Mpo, Cxcl2). Further analysis including submission of gene signatures to the LINCS database (https://www.broadinstitute.org/software/cprg/?q=node/40) will be done to identify candidate small molecules that mimic HoxA deletion in MLL-AF9.
Conclusion
Together these data support a fundamental role for the HoxA cluster in MLL-AF9 maintenance indicating dependency for this leukemia subtype which may be exploited for therapeutic benefit.
Deletion of Hoxa cluster validated by Sanger sequencing. The chromatograph (A) and sequence obtained (B) from PCR products generated from primers used to detect Hoxa cluster deletion with retention of the 5' UTR and 3' UTR regions of the Hoxa13 and Hoxa1 genes respectively.
Deletion of Hoxa cluster validated by Sanger sequencing. The chromatograph (A) and sequence obtained (B) from PCR products generated from primers used to detect Hoxa cluster deletion with retention of the 5' UTR and 3' UTR regions of the Hoxa13 and Hoxa1 genes respectively.
Deletion of the Hoxa cluster results in significant increase in survival of Cre-GFPHi AFlox-MA9 mice compared to GFPhi control mice (n=10). Significance as calculated by Log-rank (Mantel-Cox) Test is denoted *** = p<0.001.
Deletion of the Hoxa cluster results in significant increase in survival of Cre-GFPHi AFlox-MA9 mice compared to GFPhi control mice (n=10). Significance as calculated by Log-rank (Mantel-Cox) Test is denoted *** = p<0.001.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.