Abstract
*Contributed equally as first authors.
**Contributed equally as senior authors.
Recurrent mutations within EGR2, a versatile transcription factor involved in differentiation of hematopoietic cells, were recently reported in 8% of advanced-stage chronic lymphocytic leukemia (CLL) patients, where they appear to be associated with a worse outcome. EGR2 is activated through ERK phosphorylation upon B-cell receptor (BcR) stimulation, and we have previously shown that EGR2 -mutated CLL patients display altered expression of EGR2 down-stream target genes compared to wildtype (wt) patients, thereby pointing to a pathogenic role for EGR2 mutations in dysregulating BcR signaling.
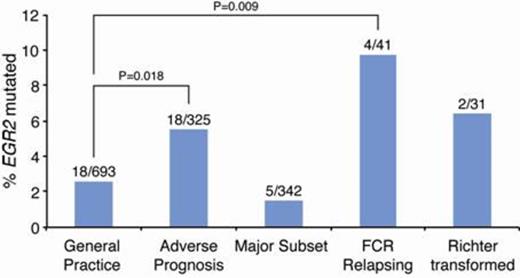
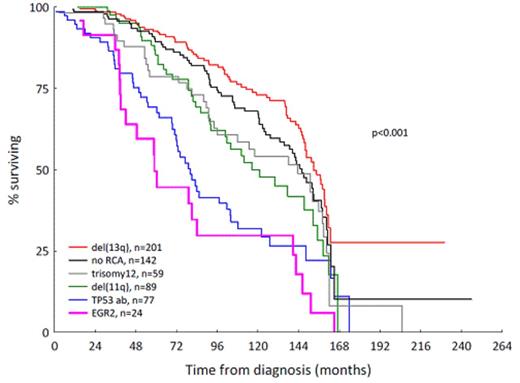
To gain further insight into the incidence and prognostic impact of EGR2 mutations in CLL, we screened samples from a well-characterized series of 1430 patients, either by Sanger sequencing (n=1019) or targeted deep-sequencing (n=370), both covering the recently reported EGR2 hotspot in exon 2. In addition, whole-exome data was available for an additional 43 patients. Different cohorts were included in our analysis ranging from 'general practice' CLL (33% IGHV-unmutated (U-CLL), 6% TP53 -aberrant (TP53abn), n=693), to adverse-prognostic CLL (89% U-CLL, 26% TP53abn, n=325), patients belonging to clinically aggressive stereotyped subsets #1-3 & #5-8 (n=342), patients relapsing after FCR therapy (n=41) and Richter transformed cases (n=31), thus reflecting the heterogeneous nature of CLL. Nineteen EGR2 mutations were detected by Sanger sequencing, while 22 additional mutations were identified with deep-sequencing using a 5% variant allele frequency (VAF) cutoff (median 39%, range 5.6-63.9%, median coverage 43,000X). With the exception of one in-frame deletion, all mutations were missense alterations located within the three zinc-finger domains. Significant enrichment of EGR2 mutations was observed in adverse-prognostic (18/325, 5.5%) and FCR-relapsing (4/41, 9.8%) CLL compared to the 'general practice' cohort (18/693, 2.6%, Figure 1A). A surprisingly low frequency was observed among clinically aggressive stereotyped subsets (5/342, 1.5%), although the cause for this observation is currently unknown. Finally, 2/31 (6.5%) cases with Richter transformation carried an EGR2 mutation. Of the 4 FCR-relapsing, EGR2 -mutated cases with available overtime samples, all demonstrated a significant expansion of the EGR2 -mutated clone at relapse (VAF-increase between 15-41%). In addition, subclonal levels of EGR2 hotspot mutations (VAF 0.5-5%) were detected in an additional 13/370 (3.5%) cases by deep-sequencing. The majority of EGR2 -mutated CLL patients (32/39, 82%) concerned U-CLL and the following aberrations co-occurred: 11q-deletions (n=10), TP53abn (n=6), NOTCH1 (n=3)or SF3B1 (n=3) mutations. EGR2 -mutated patients displayed a significantly worse overall survival compared to wt patients (median survival 59 vs. 141 months, p=0.003, using a conservative 10% VAF cutoff), and a poor outcome similar to cases with TP53abn (Figure 1B). In multivariate analysis (n=583), EGR2 status remained an independent factor (p=0.038), along with stage (p=0.048) and IGHV status (p<0.0001), while TP53abn and del(11q) showed borderline significant values (p=0.069 and p=0.059, respectively). To investigate the impact of EGR2 mutations in a homogeneously treated patient cohort, EGR2 mutation analysis of the UK CLL4 trial is underway. To date, 8/247 patients have been identified as EGR2 -mutated by deep-sequencing and they show a decrease of their median overall survival (42 vs. 77 months) compared to wt patients; however, this did not reach statistical significance, probably due to the low number of EGR2 -mutated cases. Final results of the UK CLL4 trial will be presented at the ASH meeting.
In summary, EGR2 -mutant cases appear to constitute a novel poor-prognostic subgroup of CLL, with mutations occurring either as disease-initiating aberrations, i.e. in cases where mutations were found in the entire clone, or as subclonal driver events linked to progressive disease. The latter is reflected by the enrichment of EGR2 mutations in aggressive CLL and the association of EGR2 mutations with an overall dismal prognosis. Considering the potential role of mutated EGR2 in altering BcR signaling, it will be particularly relevant to study the efficacy of BcR inhibitors in this patient group.
Langerak:Roche: Other: Lab services in the field of MRD diagnostics provided by Dept of Immunology, Erasmus MC (Rotterdam); InVivoScribe: Patents & Royalties: Licensing of IP and Patent on BIOMED-2-based methods for PCR-based Clonality Diagnostics.; DAKO: Patents & Royalties: Licensing of IP and Patent on Split-Signal FISH. Royalties for Dept. of Immunology, Erasmus MC, Rotterdam, NL. Schuh:Acerta Pharma BV: Research Funding. Strefford:Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.