Abstract
Recurrent somatic loss-of-function mutations in ASXL1 (Addition of sex combs-like 1) are common genetic events in a spectrum of myeloid malignancies and these alterations demarcate patients with poor outcome. ASXL1 forms a chromatin regulatory complex with the ubiquitin hydrolase BAP1 (BRCA1 associated protein-1), a protein that has been found to be transcriptionally repressed in MDS patients. These data are consistent with BAP1 having tumor suppressive activity in MDS; however, the mechanism by which disruption of the ASXL1-BAP1 axis leads to transformation is not well understood. We conditionally deleted Bap1 in the murine hematopoietic system utilizing Mx1-Cre (hereafter referred to as Bap1 KO). One hundred percent of mice with confirmed Bap1 deletion developed a fully penetrant MDS-like disease characterized by leukocytosis, anemia, and splenomegaly. Bap1 KO mice have an expansion of the granulocyte macrophage progenitor compartment (GMP; Lin- c-Kit+ Sca1- CD34+ Fcϒ+). Given the role of BAP1 in epigenetic regulation, we investigated the effect of Bap1 loss on chromatin state and transcriptional output.
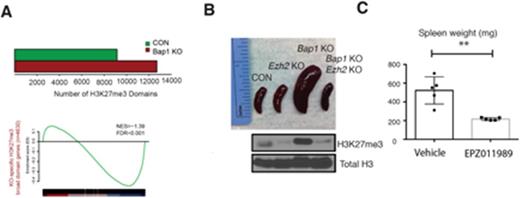
We first assessed epigenetic changes in Bap1 KO mice by performing histone mass spectometry in control and Bap1 KO hematopoietic stem and progenitor cells (HSPCs, c-Kit+ enriched). Bap1 loss increased H3K27me2/3 at the expense of H3K27me0/1. We confirmed that H3K27me3 was increased in Bap1 KO bone marrow cells by completing H3K27me3 ChIP-Sequencing in HSPCs. Enumeration of H3K27me3 peaks in Bap1 KO versus control cells indicated an increase in H3K27me3 domains (Figure A). We next overlaid RNA-Sequencing from GMP sorted Bap1 KO bone marrow cells with genes marked by H3K27me3, as indicated by ChIP-Sequencing. We found that Bap1 loss resulted in a global decrease in gene expression (68% downregulated, 657/968 genes, p-adj <0.01) and that increased H3K27me3 identified genes with reduced expression after Bap1 loss (NES=-1.39, FDR<0.001) (Figure A). Gene set enrichment analyses (GSEA) revealed that genes that were altered following depletion of Bap1 corresponded to differentiation, hematopoietic lineage specification, and proliferation pathways. Combined, these data suggest that Bap1 depletion results in increased H3K27me3 and represses gene targets implicated in normal and malignant hematopoiesis.
Given the alterations in H3K27me3 in Bap1 KO mice, we investigated whether Bap1- deficient transformation could be rescued by abrogation of PRC2-mediated gene repression. We developed a genetic model with compound deletion of Bap1 and Ezh2, the catalytic component of the PRC2 complex. Co-deletion of Bap1 and Ezh2 resulted in a phenotypic rescue of Bap1 KO associated splenomegaly (spleen weights, Bap1 KO avg. 541.6 mg, Bap1/Ezh2 KO avg. 157.0 mg, p<0.005) (Figure B), leukocytosis (white blood cells counts, Bap1 KO avg. 51 K/uL, Bap1/Ezh2 KO avg. 8 K/uL, p <0.005), anemia (hematocrit, Bap1 KO avg. 28.2%, Bap1/Ezh2 KO avg. 46.0%, p<0.005). Importantly, the increased H3K27me3 levels in Bap1 KO mice were reduced in Bap1/Ezh2 KO mice (Figure B), suggesting that loss of Bap1 leads to Ezh2-dependent malignant transformation.
EZH2 small molecule inhibitors have proven effective in EZH2-dependent models of B cell lymphoma. To determine if Ezh2 inhibition was efficacious in the setting of Bap1 loss, we treated a cohort of Bap1 KO mice with either vehicle (NaCMC) or 500 mg/kg EPZ011989, an EZH2 inhibitor with in vivo activity. Treatment of Bap1 KO mice for 16 days resulted in significant reduction of splenomegaly (spleen weights, vehicle avg. 522.0, EPZ011989 treated avg. 216.2, p<0.005) (Figure C) and anemia (white blood cell counts, vehicle avg. 61.7 K/uL, EPZ011989 treated avg. 14.5 K/uL, p <0.005), consistent with the phenotype of our genetic Bap1/Ezh2 compound deletion model. These data suggest that decreased BAP1 expression could serve as a biomarker for sensitivity to EZH2 inhibition.
Knutson:Epizyme, Inc: Employment. Campbell:Epizyme, Inc: Employment. Keilhack:Epizyme: Employment, Equity Ownership. Melnick:Janssen: Other: Research; ROCHE: Other: Research; Genentech: Speakers Bureau; Celgene: Consultancy; Eli Lilly: Consultancy; Epizyme: Consultancy. Armstrong:Epizyme, Inc: Consultancy. Levine:Foundation Medicine: Consultancy; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; Loxo Oncology: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.