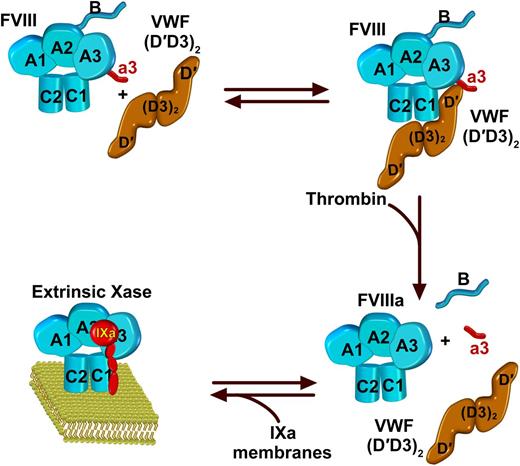
In this issue of Blood, back-to-back (dos-à-dos) papers by Chiu et al1 and Yee et al2 present complementary findings of structural investigations into the interaction between factor VIII (FVIII) and von Willebrand factor (VWF). The binding of FVIII to VWF contributes in a major way to the regulation of hemostasis.
FVIII is a heterodimer with a domain structure of A1-A2-B and A3-C1-C2. In the standard view and in accordance with crystal structures, they are illustrated with the polypeptide chain running clockwise from A1 to C2. FVIII circulates in complex with VWF. Binding to VWF is approximated by its dimeric D′D3 fragment as illustrated, with major contacts between D′ and the C1 domain. Additional interactions occur with the A3 domain and the a3 acidic peptide as well as the C2 domain. Thrombin cleavage of FVIII liberates the a3 peptide and the B domain, resulting in the dissociation of the resultant FVIIIa from VWF. FVIIIa is then available to interact with membranes and FIXa to form the extrinsic Xase complex of coagulation.
FVIII is a heterodimer with a domain structure of A1-A2-B and A3-C1-C2. In the standard view and in accordance with crystal structures, they are illustrated with the polypeptide chain running clockwise from A1 to C2. FVIII circulates in complex with VWF. Binding to VWF is approximated by its dimeric D′D3 fragment as illustrated, with major contacts between D′ and the C1 domain. Additional interactions occur with the A3 domain and the a3 acidic peptide as well as the C2 domain. Thrombin cleavage of FVIII liberates the a3 peptide and the B domain, resulting in the dissociation of the resultant FVIIIa from VWF. FVIIIa is then available to interact with membranes and FIXa to form the extrinsic Xase complex of coagulation.
The <10−9 M binding affinity for this interaction and the normal concentration of VWF in blood ensures that all FVIII circulates bound to VWF.3 VWF binding is a major determinant of FVIII stability in plasma, as type 2N mutations in VWF that impair FVIII binding are also associated with decreased FVIII levels.4 Recent advances in structure-activity relationships in VWF indicate that the dimeric D′D3 region of this multidomain and polymerized protein is sufficient to bind FVIII with high affinity and elevate FVIII levels in VWF-deficient mice.5 From the perspective of FVIII, biophysical studies in the late 1980s established much of what is widely accepted.6 All VWF-binding function resides in the light chain of FVIII (A3-C1-C2; see figure), and cleavage of the a3 acidic peptide at the N terminus of the A3 domain, for all practical purposes, abolishes VWF binding.7 Cleavage and release of the a3 peptide, associated with the activation of FVIII by thrombin, is unimpeded by bound VWF and releases FVIIIa to assemble into membrane-bound extrinsic Xase8 (see figure). Beyond stabilizing FVIII in plasma, the functional significance of this set of reactions lies in the fact that VWF and membranes are mutually exclusive ligands for FVIII.9 Membrane-dependent assembly of the Xase complex requires its proteolytically controlled release from VWF.7-9 Since these early studies, peptide studies and analysis of antibody epitopes and patient mutations have implicated the acidic a3 region and both C1 and C2 domains in VWF binding.10-12 The outstanding question has always been how such distant regions on opposite faces of FVIII could contribute to VWF binding and its controlled release by cleavage of the a3 peptide. These seemingly disparate ideas are now addressed in 2 elegant tours de force that employ low-resolution imaging techniques and high-resolution hydrogen-deuterium exchange (HDX) to reveal the basis for the binding of FVIII to VWF.
In the first study, collaborating investigators from Harvard Medical School and Biogen report on low-resolution single-molecule imaging by electron microscopy complemented by HDX to map the interface between FVIII and the VWF dimeric D′D3 fragment.1 The authors find considerable variability in the shape profiles of negatively stained electron microscopy images of the complex. Despite the implied flexibility in the D′D3 region, they note a consistent contribution of the C1 domain in mediating binding, but with some images implicating additional contacts with the C2 domain. These ideas are borne out by deuterium exchange with the polypeptide backbone impressively performed with coverage over the entire FVIII molecule both free and bound to the D′D3 dimer. They find a series of exchange hot spots in the C1 domain clustered in the vicinity of patient mutations that affect VWF binding. They also find areas of altered exchange in the C2 and A3 domains including the acidic a3 region. The major conclusions are that the C1 domain is the principal contributor to the binding of FVIII to the D′D3 fragment with additional or secondary contributions from the C2 and A3 domains including the a3 peptide.
In the second study, collaborating investigators at the University of Michigan address the same problem using low-resolution molecular envelopes reconstructed from single-particle electron microscopy studies.2 This second study not only addresses the interaction between the 2 proteins but also provides expanded insights into the disposition of both the dimeric D′D3 fragment as well as FVIII. Their image analyses reveal an antiparallel D3 dimer with the flexible D′ region projecting away from the dimer. The most extensive contacts are between the D′ region and one face of the C1 domain on FVIII extending toward the A3 domain and the a3 polypeptide region (see figure). The D3 dimer core extends across the base of the C1 and C2 domains with minimal contacts, interpreted to reflect additional weaker interactions. Rigid body fitting of the FVIII and D′ structures into the envelope suggest displacements of the C domains in comparison with their disposition in the x-ray structure. The principal conclusions from this study are that interaction between the C1 domain and the D′ region is the main determinant of the interaction between FVIII and VWF and that conformational plasticity in the interacting regions coordinates their high-affinity interaction.
The 2 studies reconcile the principal features associated with the binding of FVIII to VWF established by biochemical studies and the functional effects of C1 domain mutations in patients. The marked concordance in the conclusions arrived at by the 2 independent studies using different techniques is impressive and sheds new light on an important protein-protein interaction with major regulatory consequences for hemostasis. The details of the interaction between FVIII and VWF uncovered in the 2 papers provide a major biochemical advance with the potential for revealing new strategies for translation to the clinic as the field increasingly turns to long-acting FVIII variants for the treatment of hemophilia.
Conflict-of-interest disclosure: The author declares no competing financial interests.