Key Points
Defibrotide improves day +100 survival and CR in patients with VOD and MOF compared with a historical control.
The historical control selection methodology offers a novel approach for investigation of a life-threatening orphan disease.
Abstract
Hepatic veno-occlusive disease (VOD), also called sinusoidal obstruction syndrome (SOS), is a potentially life-threatening complication of hematopoietic stem cell transplantation (HSCT). Untreated hepatic VOD/SOS with multi-organ failure (MOF) is associated with >80% mortality. Defibrotide has shown promising efficacy treating hepatic VOD/SOS with MOF in phase 2 studies. This phase 3 study investigated safety and efficacy of defibrotide in patients with established hepatic VOD/SOS and advanced MOF. Patients (n = 102) given defibrotide 25 mg/kg per day were compared with 32 historical controls identified out of 6867 medical charts of HSCT patients by blinded independent reviewers. Baseline characteristics between groups were well balanced. The primary endpoint was survival at day +100 post-HSCT; observed rates equaled 38.2% in the defibrotide group and 25% in the controls (23% estimated difference; 95.1% confidence interval [CI], 5.2-40.8; P = .0109, using a propensity-adjusted analysis). Observed day +100 complete response (CR) rates equaled 25.5% for defibrotide and 12.5% for controls (19% difference using similar methodology; 95.1% CI, 3.5-34.6; P = .0160). Defibrotide was generally well tolerated with manageable toxicity. Related adverse events (AEs) included hemorrhage or hypotension; incidence of common hemorrhagic AEs (including pulmonary alveolar [11.8% and 15.6%] and gastrointestinal bleeding [7.8% and 9.4%]) was similar between the defibrotide and control groups, respectively. Defibrotide was associated with significant improvement in day +100 survival and CR rate. The historical-control methodology offers a novel, meaningful approach for phase 3 evaluation of orphan diseases associated with high mortality. This trial was registered at www.clinicaltrials.gov as #NCT00358501.
Introduction
Hepatic veno-occlusive disease (VOD), also called sinusoidal obstruction syndrome (SOS), is a potentially fatal complication of allogeneic or autologous hematopoietic stem cell transplantation (HSCT). VOD/SOS is clinically characterized by painful hepatomegaly, jaundice, rapid weight gain, fluid retention, and ascites.1-3 The overall incidence of VOD/SOS in patients receiving HSCT has been estimated to be 13.7% (range, 0% to 62.3%).4 Endothelial cell damage, triggered by cytotoxic chemotherapy and a prothrombotic-hypofibrinolytic state, is critical to the pathophysiology of VOD/SOS. Hepatic VOD/SOS with multi-organ failure (MOF), also known as multi-organ dysfunction, or “severe VOD,” has historically been defined by VOD/SOS with pulmonary dysfunction and/or renal dysfunction. Historical literature has established a >80% chance of mortality among well-defined patient populations with VOD/SOS and MOF.4 Although HSCT practice is changing rapidly, VOD/SOS with MOF is still a significant problem, even among patients undergoing reduced intensity conditioning and especially in the allogeneic setting.5,6
Defibrotide, a sodium salt of complex single-stranded oligodeoxyribonucleotides derived from porcine mucosal DNA, is approved in the European Union for the treatment of patients >1 month of age with severe hepatic VOD/SOS following HSCT.7-11 Preclinical data suggest that defibrotide stabilizes endothelial cells by reducing endothelial-cell activation and by protecting endothelial cells from further damage, resulting in the restoration of the thrombo-fibrinolytic balance.12-16
The primary objective of this phase 3 study was to demonstrate the efficacy of defibrotide 25 mg/kg per day in patients with hepatic VOD/SOS with MOF. Efficacy was assessed as the primary end point of difference of survival rate at day +100 post-HSCT between the 2 groups, as well as the secondary end points of difference of complete response (CR) by day +100 post-HSCT and survival at day +180 post-HSCT in patients receiving defibrotide vs historical controls.
Given the highly consistent and promising results for defibrotide treatment of hepatic VOD/SOS with MOF,7-10,17 and the lack of any other effective therapy for this life-threatening disease, it was the unanimous view of the investigators, as well as the determination of their respective institutional review boards, that a placebo-controlled study was incompatible with clinical equipoise. The data presented here are based on the final statistical analysis plan (using day +100 survival as the primary efficacy end point) and on analyses provided to the US Food and Drug Administration as part of a New Drug Application in 2015.
Patients and methods
Patients
The study was conducted at 35 centers in the United States, Canada, and Israel following approval by regulatory authorities and institutional review boards. The study included pediatric (≤16 years) and adult patients with severe hepatic VOD/SOS with MOF post-HSCT. In this study, hepatic VOD/SOS was defined by Baltimore diagnostic criteria (bilirubin ≥2 mg/dL by day +21 post-HSCT, and ≥2 of: ascites, weight gain ≥5%, or new onset or worsening hepatomegaly).18 Severe VOD/SOS was defined as VOD/SOS with advanced MOF (ie, renal and/or pulmonary dysfunction by day +28 post-HSCT). Renal dysfunction was defined as (1) serum creatinine ≥3× the value at admission to the HSCT unit for conditioning or ≥3× the lowest value during conditioning before HSCT (whichever was lower), or (2) creatinine clearance or glomerular filtration rate ≤40% of admission value, or (3) dialysis dependence. Pulmonary dysfunction was documentation of oxygen saturation ≤90% on room air or requirement for oxygen supplementation/ventilator dependence.
Exclusion criteria were preexisting liver cirrhosis, prior solid organ transplant, dialysis dependence at the time of HSCT, oxygen dependence during conditioning, and hemodynamic instability (requirement for multiple pressors or inability to maintain mean arterial pressure with single-pressor support). Concomitant use of medications increasing hemorrhagic risk (eg, heparin) was not permitted in the defibrotide group, with the exception of heparin flushes for centrally placed catheter patency or in the in-flow circuit for patients on continuous veno-venous hemodialysis, which do not increase bleeding risk.
The historical-control cohort patients met the same entry criteria, having an unequivocal diagnosis of VOD/SOS with MOF as adjudicated by an independent medical review committee (MRC) blinded to patient outcome, and having undergone HSCT ≥6 months prior to the first use of defibrotide at the participating institution.
The intent-to-treat population was defined as all patients in the defibrotide group and the historical controls. The safety population consisted of patients who received ≥1 dose of defibrotide and all the historical controls.
Patients in the defibrotide group, or their designated proxies, provided voluntary written informed consent prior to receiving treatment. For the historical controls, any patient who had refused the use of their data in research was excluded from the screening process. Procedures were conducted in accordance with institutional and national ethical standards and the Declaration of Helsinki.
Study design
This was a historically controlled, multicenter, open-label, phase 3 study where defibrotide-treated patients were enrolled from July 2006 to June 2008. In this trial, the defibrotide-treated patients were enrolled prospectively according to the definition of hepatic VOD/SOS with MOF in the study protocol as proposed by the authors, whereas the historical controls were selected by an independent MRC via retrospective review using the same criteria as for the treated patients.
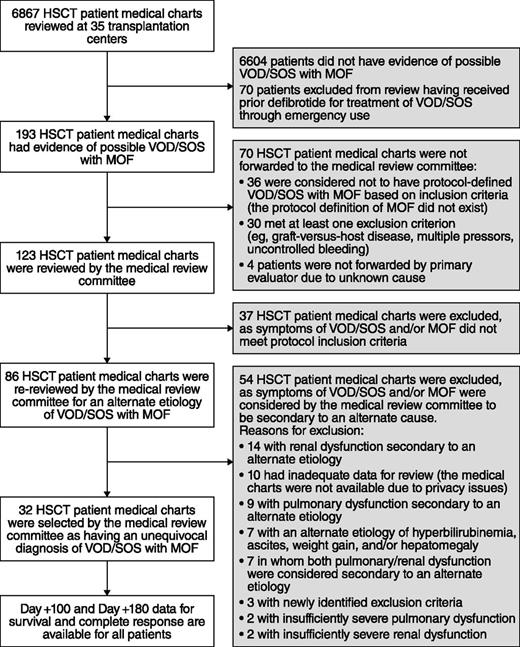
To identify a valid historical-control group, 6867 medical charts of HSCT patients hospitalized from January 1995 to November 2007 were sequentially reviewed at participating centers. Screening of medical charts began with a date specific to each center, with the start date selected 6 months prior to first approved use of defibrotide at that site (either as a clinical protocol or emergency use), moving in reverse chronological order thereafter to provide the most contemporaneous experience. Cases meeting protocol inclusion criteria were forwarded to the independent MRC, which comprised 2 expert hematologists experienced in adult and pediatric HSCT. The MRC received redacted medical charts, case report forms for inclusion criteria, and a prepared narrative; data stopped on the day the case met eligibility parameters (with the MRC blinded to outcome to minimize bias). Only the blinded MRC was in a position to decide if the symptoms were specific to VOD/SOS or whether an exclusion criterion was present. This ensured accurate and comprehensive assessments of each case, and an unequivocal diagnosis of hepatic VOD/SOS with MOF in accordance with the protocol.
Study objectives
The primary objective of this study was to demonstrate the efficacy of defibrotide in patients with hepatic VOD/SOS with MOF, in terms of difference of survival at day +100 post-HSCT in the defibrotide group vs the historical-control group. Secondary efficacy objectives included the difference of CR rate by day +100 post-HSCT and survival at day +180 post-HSCT. Incidence of adverse events (AEs) was compared between the arms.
A CR by day +100 was defined as resolution of parameters used to document VOD/SOS with organ dysfunction within a window of ± 14 days, and within the time of study entry until on or before day +100. Resolution parameters included: total bilirubin <2 mg/dL; serum creatinine <1.5× baseline or meeting upper limit of normal based on patient’s age, creatinine clearance/glomerular filtration rate >80% of initial value, and dialysis independence (for resolution of renal dysfunction); and oxygen saturation >90% on room air, no supplemental oxygen required, and ventilator-independence (for resolution of pulmonary dysfunction).
Treatment
Defibrotide was administered IV at 25 mg/kg per day in 4 divided doses, each infused over 2 hours every 6 hours, for a minimum of 21 days. Treatment was to continue beyond 21 days until resolution of VOD/SOS or until the patient’s discharge from the hospital.
AEs were coded using the Medical Dictionary for Regulatory Activities version 10.1, and for the defibrotide group, they were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.19 Defibrotide was discontinued if significant bleeding or grade 3/4 potentially drug-related toxicity occurred; a single rechallenge was permitted once the toxicity was controlled. AEs experienced by defibrotide-treated patients were assessed for seriousness, severity, and relationship to study medication. Due to the limitations in assigning severity or seriousness to events based on retrospective chart review, the AEs of historical controls were not categorized for severity or seriousness. Concomitant use of sirolimus was not recommended. Care regarding levels of tacrolimus was encouraged. Concomitant ursodiol treatment was allowed. Due to concerns about diversion of splanchnic blood flow, continuation of low-dose dopamine as part of supportive care was discouraged.
Statistical methods
The primary efficacy analysis and many of the supportive efficacy analyses were based on propensity score-adjusted estimators. Propensity scores can provide an adjustment for prognostic factors that may be unbalanced between the treatment groups in a nonrandomized setting.20 The propensity score was based on 4 prespecified factors that are prognostic of day +100 survival in this population: ventilator and/or dialysis dependency at study entry, age ≤16 vs age >16 years, allogeneic vs autologous transplant, and prior HSCT (0 vs ≥1). Patients were assigned into quintiles based on their propensity score defined as the probability of receiving defibrotide given the observed value of the 4 prognostic factors calculated using logistic regression. The survival rate for each treatment (number of patients surviving at day +100 divided by number of intent-to-treat patients) was calculated; the two-sided 95.1% confidence interval (CI) of the treatment difference (defibrotide − historical-control group), adjusted for propensity score quintile, was then calculated by the Koch et al method.21
A key secondary efficacy analysis compared day +100 CR rates between the groups, using a methodology identical to that for survival. The confidence level for CR was 95.1% in order to adjust the significance levels for the planned interim analysis. day +180 survival was analyzed using the same methodology.
The original protocol specified a sample size of 80 patients in both groups (defibrotide and historical control). However, a final historical-control group larger than 32 was not possible because continued screening at existing centers would have resulted in a less contemporaneous historical-control group (supplemental Data, available on the Blood Web site).
Results
Patients
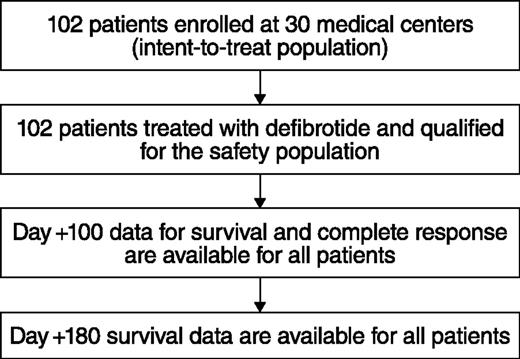
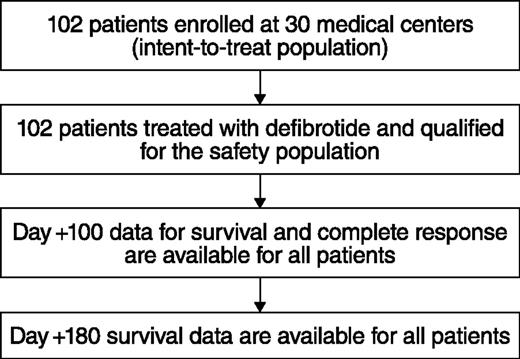
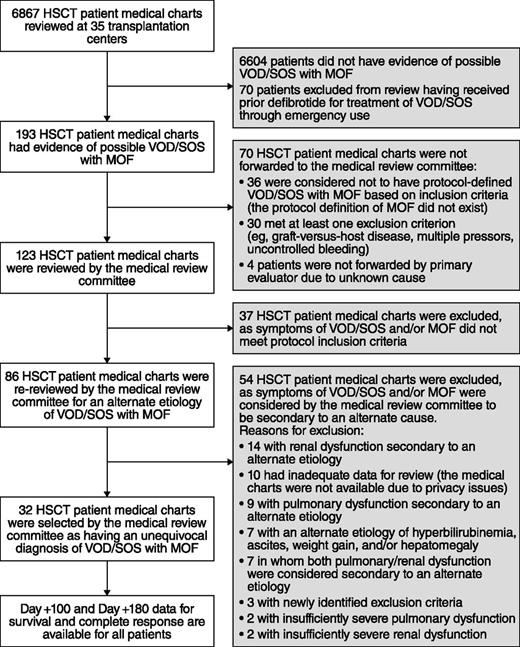
Thirty of 35 centers enrolled ≥1 patient in the total of 102 patients in the defibrotide group (Figure 1); all 35 centers initiated the historical-control selection methodology. Of the 6867 medical records screened for the historical control, the MRC reviewed 123 patients with possible hepatic VOD/SOS with MOF; patients were further excluded if they had alternate etiology for VOD/SOS-related symptoms or pulmonary and/or renal dysfunction, or if they had a protocol-defined exclusion. Thirty-two patients were subsequently selected for the historical-control group per the selection methodology (Figure 2). The majority of the historical-control patients (21/32; 66%) had a diagnosis of VOD/SOS with MOF post-HSCT in the years 2000 to 2006; the remaining 11 patients were diagnosed prior to 2000.
Demographics and baseline disease characteristics were well balanced between the groups, including all 4 baseline prognostic factors (Table 1). The groups were balanced for the other important baseline parameters, including underlying disease, graft source, conditioning regimen, myeloablative regimen, and VOD/SOS and MOF parameters. Although there were differences in graft-versus-host disease (GVHD) prophylaxis (15% of defibrotide-treated patients were started on the combination of tacrolimus and sirolimus, compared with none of the historical-control patients [Table 2]), the protocol recommended discontinuation of sirolimus, given its known endothelial toxicity and association with increased risk of VOD/SOS. Fourteen of the 15 patients receiving this doublet discontinued it upon diagnosis of VOD/SOS. Six patients were entered in violation of entry criteria (VOD/SOS diagnosis after day +21, n = 4; use of multiple pressors, n = 1; and defibrotide initiated 3 days prior to renal MOF, n = 1).
The median time to VOD/SOS diagnosis post-HSCT was 13 days in the defibrotide group (range, 1-25) and 11 days in the historical-control group (range, 4-19), with median times to MOF diagnosis of 13 and 12.5 days, respectively. Per protocol, all patients had hyperbilirubinemia. In the defibrotide group, 72% had all 3 additional VOD/SOS parameters (ascites, weight gain, and hepatomegaly), compared with 59% in the historical control.
At baseline, renal dysfunction was diagnosed in 78% of patients in the defibrotide group and 75% in the historical control. Of these, 20% and 6%, respectively, were dialysis-dependent at study entry. Pulmonary dysfunction was present in 85% and 97% of the defibrotide and historical-control groups, respectively, of which 26% and 19% were ventilator-dependent at study entry. Importantly, 64% and 72% of defibrotide-treated and historical-control patients, respectively, were diagnosed with both renal and pulmonary dysfunction.
Efficacy
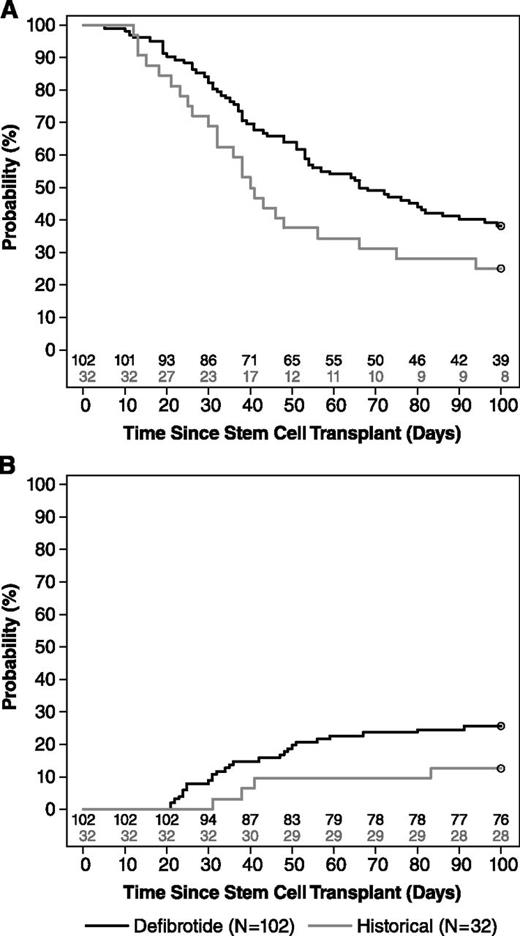
For the primary end point of survival at day +100 post-HSCT, 39 patients in the defibrotide group (38.2%) and 8 in the historical-control group (25%) were alive at day +100. The estimated between-group difference in survival was 23% (95.1% CI, 5.2-40.8; P = .0109), using the propensity-adjusted analysis (Table 3; Figure 3).
Kaplan–Meier estimate. (A) overall survival distribution and (B) time to CR in the defibrotide and historical-control groups to day +100 (supportive).
Kaplan–Meier estimate. (A) overall survival distribution and (B) time to CR in the defibrotide and historical-control groups to day +100 (supportive).
CR was reported in 25.5% of defibrotide-treated patients and 12.5% of historical controls (estimated difference adjusted for propensity score = 19%; 95.1% CI, 3.5-34.6; P = .0160). Median time to CR in defibrotide-treated patients with CR was 34.5 days (95.1% CI, 33-48.1); time to CR in the 4 historical-control patients who achieved a CR was 31 to 83 days, for a median time of 39.5 days (95.1% CI, 10.8-85.7).
CR was durable for most patients (22/26) receiving defibrotide, who continued to have a CR as of their last observation, and only 4 patients in the defibrotide group had CR end dates before day +180; all 4 patients died of sepsis or relapsed leukemia. For the historical-control group, duration of CR was either limited (2 patients with 9 and 10 days, respectively), impossible to assess (1 patient), or durable (1 patient at day +162). There was a strong association between CR and survival to day +100 post-HSCT (κ coefficient: 0.623; 95% CI, 0.467-0.779).
At day +180, 33 (32.4%) and 8 (25%) were alive in the defibrotide and historical-control groups, respectively (propensity-adjusted estimated rate difference: 16.4 [95.1% CI, −1.2-34.1]; P = .0669), but this was not a powered or prespecified end point (Table 3).
Treatment exposure and safety
The median duration of defibrotide treatment was 21.5 days (range, 1-58). Eleven patients discontinued defibrotide prematurely for a possible drug-related toxicity (10.7%).
All but 1 of the defibrotide-treated patients and all historical-control patients experienced ≥1 AE (Tables 4 and 5). Hypotension was the most common AE in both groups (39.2% with defibrotide, 50% for historical controls), and diarrhea was common in both groups (23.5% with defibrotide, 37.5% for historical controls). Overall, there was no difference in the incidence of common hemorrhagic AEs (64% and 75% of the defibrotide and historical-control groups, respectively). In the defibrotide and historical-control groups, the incidence of coagulopathy was 2% and 15.6%, and disseminated intravascular coagulation was 1% and 3.1%, respectively. Related AEs included hemorrhage and hypotension.
Pulmonary alveolar hemorrhage occurred in 11.8% and 15.6%, GI hemorrhage in 7.8% and 9.4%, and cerebral hemorrhage in 2.9% and 3.1% of the defibrotide and historical-control groups, respectively. The median time to hemorrhage onset was longer in the defibrotide group compared with the historical-control group (7 vs 3.5 days).
Sixty-five patients in the defibrotide group (64%) and 22 patients in the historical-control group (69%) experienced a fatal AE. Patients with ≥1 hemorrhagic AE leading to death in the safety analysis set included 14.7% (15 patients) in the defibrotide group and 6.3% (2 patients) in the historical-control group. For the defibrotide-treated group and historical controls, respectively, hemorrhagic AEs were GI hemorrhage (1 and 0), cerebral hemorrhage (2 and 0), intracranial hemorrhage (1 and 0), subarachnoid hemorrhage (1 and 0), pulmonary alveolar hemorrhage (7 and 2), pulmonary hemorrhage (2 and 0), and vascular disorders hemorrhage (1 and 0). The most common AE with a fatal outcome, specifically pulmonary alveolar hemorrhage, was observed with a similar incidence between the 2 groups (6.9% vs 6.3% for defibrotide and historical controls, respectively).
The percentages of patients with neutrophil engraftment by day +100 were similar between the defibrotide-treatment group and the historical-control group (87.3% vs 81.3%). Time to engraftment was slightly shorter in the defibrotide group, with a median time of 17 days compared with 21 days for the historical controls. The number of patients who had an absolute neutrophil count >0.5 × 109/L was higher in the defibrotide group compared with the historical controls (71.6% vs 43.8%).
Discussion
This study demonstrated that, compared with rigorously selected historical controls, defibrotide use in patients with hepatic VOD/SOS and advanced MOF post-HSCT was associated with a clinically meaningful improvement in survival and in rate of CR by day +100, compared with historical controls. Importantly, the day +100 survival in the historical-control group, at 25%, is similar to the rate observed in other series (<20% and <31%).4,22
Preliminary analyses from this study23 provided support for the European Commission marketing authorization for defibrotide for the treatment of severe hepatic VOD/SOS in adults and children following HSCT.11 Those analyses23 showed results in concordance with this final analysis: the percentage of patients surviving at day +100 in each group did not vary (38.2% for the defibrotide group and 25% for the historical-control group); however, given changes to baseline prognostic variables in several patients, the propensity-adjusted analysis showed an improved level of statistical significance (previously calculated to be a 95% CI difference: −32 to 3; P = .051; in the present analysis, 95.1% CI, 5.2-40.8; P = .0109). The day +100 CR rates in the defibrotide and historical-control arms in the original analysis were both slightly lower at 24% and 9%, respectively, resulting in a nearly identical P value to the present analysis (95% CI difference, 3-30; P = .015; in the present analysis, 95.1% CI, 3.5-34.6; P = .016). Many of these differences are due to specific requests from the US Food and Drug Administration, including additional data collection requirements, and an updated definition of CR in the final statistical analysis plan.
The use of the derived historical control selection methodology based on sequential chart review is, to our knowledge, unique for such an uncommon and frequently fatal HSCT complication. Although randomized controlled trials (RCTs) represent the gold standard, the historical-control methodology provides a feasible mechanism for comparison when an RCT is not possible due to ethical issues. Because recruitment for the defibrotide group and screening for the historical-control group occurred at the same medical centers, with minimal temporal disparity between the populations, there is a high degree of confidence in comparable patient management and supportive care. Of note, historical controls at any center typically received HSCT during a 1- to 2-year time span, and only 12 of 35 centers screened charts prior to 2000; therefore, 66% of the cases occurred during 2000 or later. Further, although there have been improvements in survival in the early post-HSCT period with improvements in supportive care, no improvement has been seen during this period in the treatment of VOD/SOS once MOF has developed.6
Importantly, the incidence of VOD/SOS with MOF identified by the rigorous historical control screening process was similar to registry data reported previously in the literature. In this study, 32 eligible patients met protocol criteria for VOD/SOS with MOF and were included in this study; in addition, 70 patients met these criteria but had received defibrotide and were thus excluded, for an overall incidence of 1.5% (102/6867). This is similar to data from cases recorded from 2008 to 2011 in a worldwide registry (1.2% of 8341 HSCT patients).22 Moreover, a large Japanese HSCT registry analysis of 4290 patients found a rate of severe VOD/SOS of 3.9%.24
Although the difference between groups in day +100 survival was statistically significant, day +180 survival did not reach statistical significance. Importantly, all deaths in both groups after day +100 were considered to be related to a cause other than VOD/SOS. The contribution of multiple factors other than VOD/SOS to mortality beyond day +100 confounds the use of survival at day +180 as an efficacy measure for early post-HSCT toxicity. Within the limits of this design, the temporal proximity between HSCT and onset of VOD/SOS support the use of survival at day +100 as a more clinically relevant efficacy end point for VOD/SOS than longer-term analyses.
The lower CR rate seen by day +100 post-HSCT in the defibrotide group (25.5%) compared with previous phase 2 trials in similar populations (36% and 46% for the 25 mg/kg per day group) reflects the more advanced MOF in a gravely ill patient population with a much higher incidence of ventilator and/or dialysis dependence at study entry compared with previous studies.7,8
The recommended duration of therapy in this study was ≥21 days, which also was observed as the median duration. This median and recommended duration was similar to the observed difference between median time to diagnosis (13 days) and median time to onset of CR (34.5 days), as well as median length of treatment in the phase 2 dose-finding study.8 In that phase 2 study, although the minimum duration of dosing was 14 days, the actual median length of treatment was 19.5 days in both defibrotide-treatment arms.8 This is reflected in the European Union-approved administration of defibrotide for a minimum of 21 days and continued until the symptoms and signs of severe VOD/SOS resolve.11
The type, incidence, and severity of AEs were as expected in this critically ill population. Overall, AEs did not occur at a higher incidence in the defibrotide-treatment group compared with the historical controls, and defibrotide was generally well tolerated in this very sick population. Although overall rates of hemorrhage were similar, the rate of fatal hemorrhage was somewhat higher in the defibrotide group. The overall contribution of defibrotide to fatal hemorrhagic AEs was difficult to quantify in an open-label trial, given the comorbidities and underlying high risk of bleeding in these post-HSCT patients with VOD/SOS, VOD/SOS-induced attendant liver dysfunction, and advanced MOF. Of the defibrotide-treated patients with fatal hemorrhagic AEs, comorbidities included sepsis (4 patients), infection other than sepsis (5 patients), coagulopathy (thrombotic thrombocytopenic purpura, disseminated intravascular coagulation, and coagulopathy not otherwise specified; 3 patients), GVHD (1 patient), acute respiratory distress syndrome (1 patient), and systemic inflammatory reaction (1 patient).
This study had both strengths and limitations. Although use of a historical control provided a feasible and ethically sound approach to phase 3 evaluation of defibrotide in an orphan-drug setting when an RCT is not feasible, the historical-control size limited the statistical power of the analysis in this trial. In spite of this, significance for the primary outcome was still achieved using a 95.1% CI. Care was taken to minimize selection bias and achieve a balanced patient population between the 2 groups. To account for the lack of randomization in the trial, primary efficacy analyses included propensity-score adjustments based on baseline prognostic factors of survival. Importantly, no statistically significant differences between the 4 prognostic factors were observed between the 2 groups. The only baseline characteristic that differed between the 2 groups was choice of GVHD prophylaxis, including the combination of sirolimus with tacrolimus, a doublet that has been shown to improve early survival in the context of GVHD but has been associated with a tripling of the incidence of VOD/SOS.25 Because all but 1 patient in the defibrotide arm receiving sirolimus stopped this drug upon diagnosis of VOD/SOS, any impact of this imbalance is likely to be minimal (although an unrecognized long-term effect cannot be ruled out); conversely, its effect on exacerbating endothelial injury and VOD/SOS initiation is probably substantial.
Given the poor outcome associated with hepatic VOD/SOS with MOF, future studies of defibrotide should investigate earlier intervention and efficacy in combination with other therapies,26 as well as activity in high-risk groups such as sirolimus-exposed patients,25,27 and VOD/SOS prophylaxis for patients undergoing allogeneic or high-risk autologous HSCT.28 Defibrotide has already shown efficacy in a number of prevention studies in pediatric patients, including a large randomized prospective study, and demonstrated promise both in clinically defined and genotypically characterized high-risk groups.29-32
In conclusion, use of defibrotide provided a 23% improvement in survival at day +100 post-HSCT in patients with hepatic VOD/SOS with MOF in this study using a novel, sequentially derived historical control selection methodology developed for this rare, life-threatening condition. Although HSCT practice has changed greatly over the past decades, hepatic VOD/SOS with MOF remains a very real and life-threatening complication post-HSCT, for which there are no approved therapies. In this context, defibrotide provides a promising treatment option for patients with a high unmet medical need.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge the members of the independent MRC, specifically Professors Selim Corbacioglu and Ernst Holler, for their essential role in identifying the historical controls and comprehensive chart review; all data and study monitoring members, study staff, nursing teams, and medical personnel; and in particular all participating patients and their families. Medical writing and editorial assistance for the development of this manuscript was provided by Dr Jonathan Viney (apothecom scopemedical) and The Curry Rockefeller Group, LLC, funded by Jazz Pharmaceuticals.
This study was funded by Gentium SpA, now Jazz Pharmaceuticals. N.A.K.’s research was supported by the National Cancer Institute of the National Institutes of Health (P30 CA008748). The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: P.G.R., R.J.S., R.L.H., M.I., and A.L.H. were responsible for the study conception and design. P.G.R., M.L.R., N.A.K., J.A.B., S.M., A.M.T., S.A., S.A.G., E.C.G., P.L.M., G.S., A.K., E.R.N., S.G., T.R., R.D., J.D., J.H.A., A.S., L.L., R.C., A.G., R.B., R.B.D’A., D.W., M.M., R.L.H., M.I., B.N., A.L.H., and R.J.S. were involved in the collection and assembly of data; J.M. performed statistical analysis; and all authors contributed to the provision of study materials or patients, participated in the study data analysis and interpretation, manuscript writing, and provided their final approval of this manuscript.
Conflict-of-interest disclosure: P.G.R. and R.J.S. have served on advisory committees for Gentium SpA/Jazz Pharmaceuticals; R.B.D'A. and J.M. report consultancy fees from Gentium SpA during the conduct of the study; J.D., N.A.K., P.L.M., P.G.R., T.R., and G.S. received grants from Gentium SpA during the conduct of the study; S.A.G. and A.K. have served as consultants to Jazz Pharmaceuticals; S.G. received personal fees and grants from Gentium SpA during the conduct of the study; E.C.G.’s institution received clinical research fees from Gentium during the conduct of the study; A.L.H. received personal fees from Gentium SpA during the conduct of the study; J.H.A. and P.L.M. have served on advisory committees with Jazz Pharmaceuticals; R.C. has served as an advisor to Gentium SpA and on advisory committees with Jazz Pharmaceuticals; R.L.H. and M.M. are employees of Jazz Pharmaceuticals, who in the course of employment have received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc; B.N. was an employee of Jazz Pharmaceuticals, who in the course of employment received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc; and M.I. was an employee of Gentium SpA during the conduct of the study. The remaining authors declare no competing financial interests.
Correspondence: Paul G. Richardson, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: paul_richardson@dfci.harvard.edu.