Key Points
BM FRC-like cells regulate megakaryocytic clonal expansion via CLEC-2/PDPN interactions.
CLEC-2/PDPN binding stimulates BM FRC-like cells to secrete the proplatelet formation-promoting factor, CCL5.
Abstract
Megakaryopoiesis is the hierarchical differentiation of hematopoietic stem cells into megakaryocytes. Differentiating megakaryocytes undergo maturation characterized by endomitosis and produce numerous platelets through proplatelet formation. C-type lectin-like receptor 2 (CLEC-2) is a podoplanin (PDPN) receptor mainly expressed on platelets and megakaryocytes. Deletion of platelet/megakaryocyte CLEC-2 causes thrombocytopenia in mice; however, its contribution to megakaryopoiesis remains unknown. Here, we show that megakaryopoiesis is promoted through the CLEC-2/PDPN interaction in the vicinity of arterioles in the bone marrow (BM). We have also identified PDPN-expressing BM arteriolar stromal cells, tentatively termed as BM fibroblastic reticular cell (FRC)-like cells. Platelet/megakaryocyte-specific CLEC-2 conditional knockout (cKO) mice showed a decrease in the number of immature megakaryocytes. CLEC-2 wild-type megakaryocyte expansion was augmented in vitro by the addition of recombinant PDPN, but not cKO megakaryocytes. Moreover, megakaryocyte colonies were colocalized with periarteriolar BM FRC-like cells in the BM. Coculture of megakaryocytes with BM FRC-like cells augmented megakaryocyte expansion, which was dependent upon the CLEC-2/PDPN interaction. Furthermore, we found that the CLEC-2/PDPN interaction induces BM FRC-like cells to secrete chemokine (C-C motif) ligand 5 (CCL5) to facilitate proplatelet formation. These observations indicate that a reciprocal interaction between CLEC-2 on megakaryocytes and PDPN on BM FRC-like cells contributes to the periarteriolar megakaryopoietic microenvironment in mouse BM.
Introduction
Megakaryopoiesis is a hierarchical differentiation process from hematopoietic stem cells to megakaryocytes, which culminates in platelet production.1,2 Hematopoietic precursors are committed to megakaryocyte lineages via megakaryocyte-erythroid precursors. Megakaryocyte progenitors (burst forming unit–megakaryocyte, colony forming unit–megakaryocyte [CFU-MK], and megakaryoblasts) are immature cells that can expand to form megakaryocytic clusters, a process referred to as clonal expansion. Megakaryocyte progenitors in turn undergo a remarkable endoreplicative process known as endomitosis, resulting in cytoplasmic extension with polyploidization and abundant protein synthesis.3,4 Fully matured megakaryocytes can produce numerous platelets through the elaboration of proplatelet formation (PPF).5 While differentiating from hematopoietic stem cells, megakaryocytes migrate between 2 distinct microenvironments: the endosteal niche and the vascular niche.6,7 The endosteal niche provides a proliferative microenvironment for megakaryocyte progenitors. The vascular niche consists of sinusoids and perisinusoidal stromal cells that are present in the maturational microenvironment where platelets are produced and shed into bloodstream.
C-type lectin-like receptor 2 (CLEC-2) is a multifaceted platelet receptor that plays a role in lymph node (LN) homeostasis, maintenance of vasculature integrity, and thrombus formation.8,9 CLEC-2 is not only highly expressed in platelets and megakaryocytes, but is also present at low levels in other hematopoietic cells, including neutrophils or dendritic cells.10 The endogenous ligand of CLEC-2, podoplanin (PDPN; also known as Aggrus or gp38), is highly expressed in lymphatic endothelial cells (LECs) and LN fibroblastic reticular cells (FRCs).11 Within the LNs, PDPN on FRCs maintains high endothelial venule integrity by binding to CLEC-2 on extravasated platelets and sphingosine-1-phosphate released from activated platelets.12 Alternatively, dendritic cell–derived CLEC-2 modulates LN FRC network tension by binding to FRC PDPN, subsequently increasing dendritic cell motility.13-15 This reciprocal interaction between dendritic cell CLEC-2 and FRC PDPN contributes to optimal immune responses in LNs. Moreover, platelet CLEC-2 facilitates the separation of blood/lymphatic vessels by binding PDPN on LECs during development,16 as mice deficient in CLEC-2 or PDPN show embryonic/neonatal lethality with blood-filled lymphatic vessels and severe edema due to blood-lymphatic vessel misconnections.17-21 Several research groups, including ours, have found that surviving CLEC-2–knockout (KO) neonates or megakaryocyte/platelet-specific CLEC-2 conditional KO (cKO) mice exhibit thrombocytopenia (Figure 1A)22,23 ; however, the role of CLEC-2 in thrombocytopenia pathogenesis remains to be elucidated.
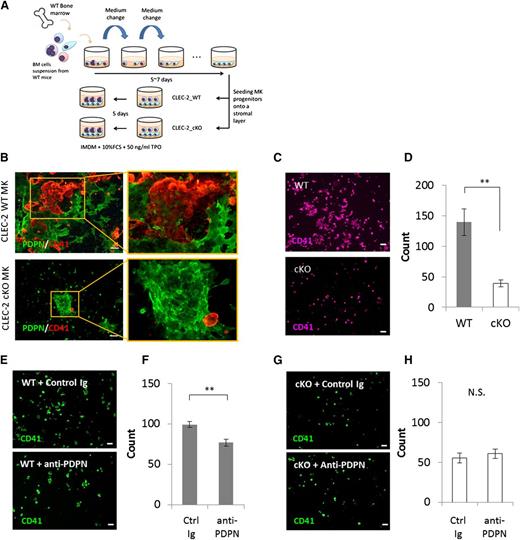
CLEC-2/PDPN interaction promoted megakaryocyte expansion. (A) Blood platelet numbers in CLEC-2 WT and cKO mice. CLEC-2 WT, n = 19; CLEC-2 cKO, n = 22. (B) CLEC-2 expression pattern during megakaryocyte differentiation. BM- or fetal liver-derived megakaryocytes were differentiated with TPO in vitro. CLEC-2 expression pattern was validated by ICC. (C) Quantification of BM megakaryocytes in CLEC-2 WT and cKO mice by FACS. The graph on the left indicates the total numbers of CD41+ megakaryocytes. The graphs in the middle and on the right show the numbers of megakaryocyte subsets, CD41+CD42b− (immature megakaryocytes) and CD41+CD42b+ (mature megakaryocytes), respectively. CLEC-2 WT, n = 8; CLEC-2 cKO, n = 11. (D-E) Quantification of megakaryocyte expansion by the MegaCult-C colony-formation assay. (D) Autonomous expansion in CLEC-2 WT and cKO megakaryocytes, n = 3 per group. (E) Stimulated expansion with recombinant PDPN (PDPN-rFC2, 20 μg/mL), n = 4 per group. The error bars reflect SEMs. *P < .05. **P < .01. N.S., a nonsignificant difference.
CLEC-2/PDPN interaction promoted megakaryocyte expansion. (A) Blood platelet numbers in CLEC-2 WT and cKO mice. CLEC-2 WT, n = 19; CLEC-2 cKO, n = 22. (B) CLEC-2 expression pattern during megakaryocyte differentiation. BM- or fetal liver-derived megakaryocytes were differentiated with TPO in vitro. CLEC-2 expression pattern was validated by ICC. (C) Quantification of BM megakaryocytes in CLEC-2 WT and cKO mice by FACS. The graph on the left indicates the total numbers of CD41+ megakaryocytes. The graphs in the middle and on the right show the numbers of megakaryocyte subsets, CD41+CD42b− (immature megakaryocytes) and CD41+CD42b+ (mature megakaryocytes), respectively. CLEC-2 WT, n = 8; CLEC-2 cKO, n = 11. (D-E) Quantification of megakaryocyte expansion by the MegaCult-C colony-formation assay. (D) Autonomous expansion in CLEC-2 WT and cKO megakaryocytes, n = 3 per group. (E) Stimulated expansion with recombinant PDPN (PDPN-rFC2, 20 μg/mL), n = 4 per group. The error bars reflect SEMs. *P < .05. **P < .01. N.S., a nonsignificant difference.
In the present study, we demonstrated that immature megakaryocytes were reduced in the bone marrow (BM) of CLEC-2 cKO mice. In vitro experiments revealed that recombinant PDPN augmented megakaryocytic clonal expansion via binding to CLEC-2. We also identified PDPN-expressing periarteriolar pericytes in the BM, which were tentatively termed as BM fibroblastic reticular cells (BM FRC-like cells). In the BM, megakaryocytic colonies developed in the vicinity of arterioles by associating with BM FRC-like cells. In coculture of megakaryocytes with BM FRC-like cells, CLEC-2 cKO megakaryocyte expansion was significantly decreased when compared with CLEC-2 wild-type (WT) megakaryocytes, which indicated that BM FRC-like cells augmented megakaryocyte expansion via CLEC-2/PDPN interaction. In addition, CLEC-2 binding to BM FRC-like cells via PDPN induced the secretion of chemokine (C-C motif) ligand 5 (CCL5; regulated on activation normal T expressed and secreted [RANTES]), which potentiates megakaryocyte PPF. We propose a novel periarteriolar megakaryocytic microenvironment that involves reciprocal CLEC-2/PDPN interactions between megakaryocytes and BM FRC-like cells.
Materials and methods
Mice
Mice were bred and maintained under standard conditions. This study was approved by the animal care and use committee at the University of Yamanashi. Megakaryocyte and platelet-specific CLEC-2–cKO mice (Pf4-Cre, Clec1bflox/flox) were generated as described.18,19 C57BL/6 mice were purchased from Japan CLEA (Tokyo, Japan) or Japan Charles River (Kanagawa, Japan).
BM megakaryocyte progenitor separation
BM cells were harvested from femurs and tibias of young mice (∼7-12 weeks old). Flushed BM was suspended with cold Iscove modified Dulbecco medium (IMDM; Life Technologies) supplemented with antibiotics and mixed well by pipetting. After passing the cells through a 40-μm strainer (BD Biosciences), megakaryocyte progenitors were separated by Lin+ depletion, as previously described.24 Briefly, BM cells were stained with specific antibodies for hematopoietic markers (CD4, CD8, B220, Thy1.2, TER-119, Ly-6G, CD11b, F4/80, anti-neutrophil antibody, and CD71). Stained Lin+ cells were depleted using sheep anti-rat immunoglobulin G (IgG) polyclonal antibody (pAb)-conjugated magnetic beads (Dynabeads; Life Technologies). Live cell numbers were counted at least twice using a TC20 automatic cell counter (Bio-Rad, Hercules, CA) with trypan blue staining.
BM stromal cell separation
BM stromal cells were separated as previously described.25 Briefly, flushed BM cells were suspended in IMDM containing 10% fetal calf serum (FCS) and antibiotics and seeded into 12-well plates or cell-culture dishes. Culture media was daily changed to remove nonadhesive cells for 5 to 7 days until adhesive BM stromal cells formed a monolayer.
In vitro megakaryocyte differentiation and coculture with BM stromal cells
BM progenitors or embryonic day 13.5 fetal liver cells were cultured in IMDM supplemented with 10% FCS, antibiotics, and 50 ng/mL thrombopoietin (TPO) for 5 days to induce megakaryocyte differentiation. At culture day 3, GM6001 (50 μM final concentration; Millipore, Billerica, MA), a broad-range matrix metalloproteinase inhibitor, was added to the culture medium to suppress the shedding of surface proteins from megakaryocytes. For the coculturing experiments, BM progenitors (1.0 × 105 cells per mL) were cultured on a BM stromal cell layer in 12-well plates.
Colony-formation assay
BM megakaryocyte progenitors (2.2 × 106/mL) were applied to MegaCult-C (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 50 ng/mL TPO, 10 ng/mL interleukin-3 (IL-3), and 20 ng/mL IL-6 in double-chamber slides (Nunc, Rochester, NY). Megakaryocytic colonies (CFU-MK) were detected by acetylcholinesterase staining. Counter staining was performed with Harris’ hematoxylin (Sigma-Aldrich). We defined CFU-MK as colonies tightly contacting at least 3 acetylcholinesterase-positive megakaryocytes according to the manufacturer’s instructions.
Immunohistochemistry
Before enucleation of femurs, we perfused 8-week-old mice with 4% paraformaldehyde (PFA). Long bones were then fixed with 4% PFA for 3 hours and decalcified overnight with K-CX solution (FALMA, Tokyo, Japan). After washing with diluted water, long bones were cut into longitudinal or transverse pieces. Bone pieces were subsequently incubated in 30% sucrose for cryoprotection. Treated tissues were embedded in OCT (Tissue-Tek, Sakura, Japan) at −80°C. Frozen sections were prepared as previously described.26 Frozen BM sections sliced at 10 μm were blocked with phosphate-buffered saline (PBS) containing 3% bovine serum albumin and 2% goat serum. Anti-CLEC-2 (2A2B10, 1/200 dilution), anti-CD41 (MWReg30, 1/500 dilution), anti-PDPN (8.1.1, 1/200 dilution) were diluted with blocking reagent and incubated for 1 hour at room temperature. Anti-Syrian hamster Alexa 488 conjugate (for PDPN, 1/1000 dilution) and anti-rat Alexa 546 conjugate (for CD41, 1/2000 dilution) were used as secondary antibodies. 4,6 Diamidino-2-phenylindole (DAPI) was used to counterstain the nucleus. After inclusion with Mounting PermaFluor (Thermo Fisher, Waltham, MA), sections were observed using an inverted fluorescence microscope (IX71; Olympus) or inverted confocal fluorescence microscope (FV1000; Olympus).
Immunocytochemistry
Cells were plated and cultured onto φ15-mm growth cover glasses (Thermo) in 12-well culture plates. Cells were fixed with final 4% PFA. For megakaryocytes and stromal cell cocultures, we carefully pipetted drops of fixative into the wells and fixed cells for 1 hour to preserve cell-cell contacts. Cells were then permeabilized with 0.1% Triton X-100 in PBS for 10 minutes if necessary. After washing with PBS, cells were blocked with PBS containing 3% bovine serum albumin and 2% goat serum, and incubated with antibodies of anti-PDPN (8.1.1, 1/200 dilution), anti-CD41 (MWReg30, 1/500 dilution), anti-CLEC-2 (2A2B10, 1/200 dilution), anti-CD45 (30-F11, 1/200 dilution), anti-osteocalcin rabbit pAb (1/1000 dilution), or anti-N-cadherin rabbit pAb (1/400 dilution). The secondary antibodies used were anti-Syrian hamster Alexa 488 conjugate (for PDPN, 1/1000 dilution), anti-rat Alexa 546 conjugate (for CD41, 1/2000 dilution), and anti-rabbit Alexa 546 conjugate (for N-cadherin, 1/2000 dilution). DAPI was used as a nuclear counterstain. After mounting the sections with Mounting PermaFluor (Thermo Fisher), they were observed using an inverted fluorescence microscope (IX71; Olympus). Acquired images were quantitatively analyzed by Image J 1.46r software (http://rsb.info.nih.gov/ij/).
PPF assays
PPF assays were performed as previously described.27 In brief, mouse fetal liver cells were harvested from embryonic day 13.5 embryos and cultured in IMDM supplemented with 10% FCS, antibiotics, and 50 ng/mL TPO. The next day, supernatant hematopoietic cells were transferred to new wells to remove adherent cells. At culture day 4, proplatelet-forming megakaryocytes were counted using an IX71 inverted microscope (Olympus).
In proplatelet formation assay with conditioned medium, conditioned medium was added at culture day 2. To prepare conditioned medium, 20 μg/mL recombinant rFC2 (control protein) or mouse CLEC-2 rFC2 fusion protein was added to BM stromal cell layers containing BM FRC-like cells. After 48 hours, the culture supernatant was centrifuged at 1000g for 5 minutes, and the harvested supernatant preserved at −80°C until analysis.
Antibody array
The concentrations of cytokines and chemokines in conditioned medium were analyzed using the Proteome Profiler Mouse Cytokine Array Panel A (ARY006; R&D Systems).28 Conditioned medium (1 mL) was applied to the array membrane according to the manufacturer’s instructions. We used ECL Prime (GE Healthcare, Little Chalfont, United Kingdom) as a horseradish peroxidase substrate, and chemiluminescence signals were detected with an LAS4000 Mini (GE Healthcare). Quantification analysis was performed using Image J 1.46r software.
Statistics
All quantitative data are shown as the mean ± standard error of the mean (SEM). Representative data from at least 3 independent experiments are shown in the immunohistochemistry (IHC) and immunocytochemistry (ICC) photographs. Comparisons between 2 groups were analyzed using unpaired the Student t test or Mann-Whitney U test. Comparisons in multiple groups were examined by Tukey 1-way analysis of variance. Independence in a contiguity table was examined by χ2 test. Statistical analyses were carried out in GraphPad Prism 5.
Results
Interaction between CLEC-2 and PDPN accelerates megakaryocytic clonal expansion
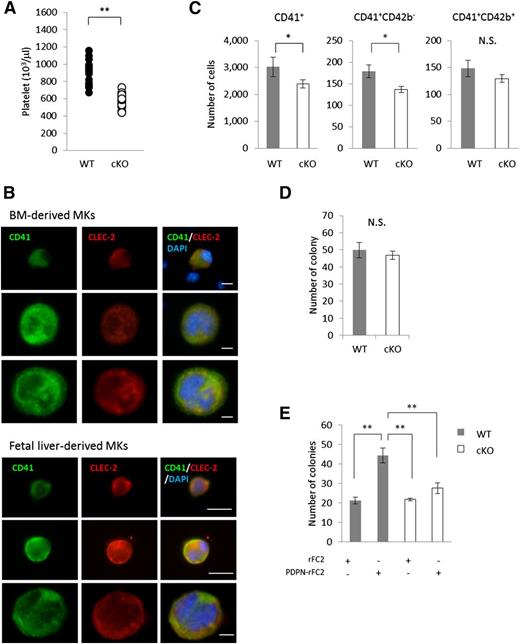
To first verify the presence of the megakaryocyte-specific genetic deletion of CLEC-2 in the BM, we assessed its expression in the femur BM of CLEC-2 WT and cKO mice. As expected, histologic analysis demonstrated that CLEC-2 cKO mice were completely devoid of CLEC-2 expression within the BM compartment (supplemental Figure 1, available on the Blood Web site). Moreover, we also measured CLEC-2 expression during megakaryocyte differentiation from the early to the late stages (Figure 1B). CLEC-2 expression was detected from CD41+ mononuclear small megakaryocytes to mature large polyploidic megakaryocytes. These findings indicated that megakaryocytes expressed CLEC-2 during the early phase of differentiation.
Interestingly, an analysis of BM megakaryocyte numbers in WT and CLEC-2 cKO mice revealed that CLEC-2 cKO mice exhibited a marked reduction in megakaryocyte-lineage numbers compared with their WT counterparts, particularly in immature megakaryocytes (CD41+CD42b−; Figure 1C). These observations suggested that CLEC-2 is likely involved in the clonal expansion of megakaryocyte progenitors in the BM; however, we found no differences in the in vitro autonomous clonal expansion of megakaryocyte progenitors between CLEC-2 WT and cKO mice (Figure 1D). We next investigated the effect of recombinant PDPN (PDPN-rFC2) treatment on megakaryocyte progenitor expansion and found that PDPN-rFC2 enhanced the expansion of WT megakaryocytes, but not that of CLEC-2 cKO megakaryocytes (Figure 1E). These data indicated that the CLEC-2/PDPN interaction accelerates megakaryocytic clonal expansion.
Megakaryocytic colonies are developed in association with PDPN-expressing arteriolar pericytes in the BM
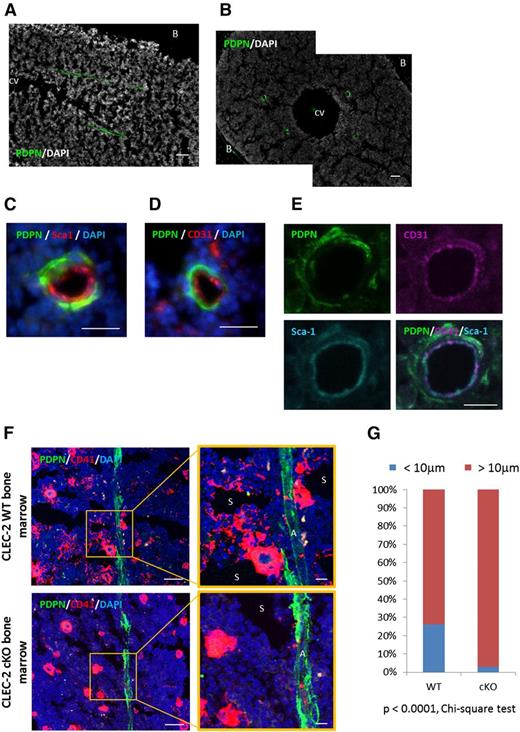
Next, we investigated whether PDPN-expressing stromal cells existed within the BM compartment. A histologic analysis revealed the presence of PDPN along vascular structures (Figure 2A-B; supplemental Figure 2). The BM vasculature is typically divided into 3 subtypes: arterioles (CD31+Sca-1+), sinusoids (CD31+Sca-1−), and central veins.29 The central vein can be easily identified by its large structure, and it is PDPN−. Next, we focused on other BM sinusoids and arterioles. IHC showed that PDPN+ cells surrounded the CD31+Sca-1+ vasculature (Figure 2C-E), indicating that PDPN+ stromal cells were analogous to BM arteriole pericytes. Furthermore, we found that mature large megakaryocytes and immature megakaryocytic colonies (CD41+ clusters) were adjacent to PDPN+ stromal cells in the WT BM (Figure 2F). In contrast, in the CLEC-2 cKO BM, megakaryocytes or immature megakaryocytic colonies were rarely detected in the periarteriolar space. We also calculated the percentage of megakaryocytes and megakaryocytic colonies associated with PDPN+ stromal cells at periarteriolar sites (Figure 2G). CD41+ nucleated cells and colonies localized within 10 μm from PDPN+ stromal cells were defined as megakaryocytes or immature megakaryocytic colonies associated with arterioles. In the BM of WT and CLEC-2 cKO mice, the percentages of megakaryocytes and related colonies localized at the periarteriolar space were 26.09% and 3.00%, respectively (P < .0001, χ2 test). These observations suggest that PDPN-expressing stromal cells contribute to megakaryopoiesis within the periarteriolar space.
Megakaryocytic colonies developed in contact with PDPN-expressing arteriolar pericytes in the BM. (A-B) Representative images of longitudinal (A) and transverse (B) femurs with anti-PDPN and DAPI staining. (C-E) Representative images of BM vasculature ensheathed in PDPN+ stromal cells staining with PDPN/Sca-1/DAPI (C), PDPN/CD31/DAPI (D), and PDPN/CD31/Sca-1 (E). (F) Representative IHC images of the CLEC-2 WT and CLEC-2 cKO BM staining with CD41, PDPN, and DAPI. In CLEC-2 WT BM, CD41+ megakaryocyte clusters located adjacent to PDPN+ stromal cells at periarteriolar sites in the BM. (G) Percentage of megakaryocytes and megakaryocytic colonies which associated with PDPN+ stromal cells at periarteriolar space in the CLEC-2 WT and cKO BM. CD41+ nucleated cells and colonies localizing within 10 μm distance from PDPN+ stromal cells were defined as the megakaryocytes or immature megakaryocytic colonies associated with arteriole. Data were obtained using frozen BM sections from 3 individual CLEC-2 WT or CLEC-2 cKO mice. Scale bars, 100 μm for A and B; 50 μm for C, D, and F; 10 μm for E and magnified image in panel F. A, arteriole; B, bone; CV, central vein; S, sinusoid.
Megakaryocytic colonies developed in contact with PDPN-expressing arteriolar pericytes in the BM. (A-B) Representative images of longitudinal (A) and transverse (B) femurs with anti-PDPN and DAPI staining. (C-E) Representative images of BM vasculature ensheathed in PDPN+ stromal cells staining with PDPN/Sca-1/DAPI (C), PDPN/CD31/DAPI (D), and PDPN/CD31/Sca-1 (E). (F) Representative IHC images of the CLEC-2 WT and CLEC-2 cKO BM staining with CD41, PDPN, and DAPI. In CLEC-2 WT BM, CD41+ megakaryocyte clusters located adjacent to PDPN+ stromal cells at periarteriolar sites in the BM. (G) Percentage of megakaryocytes and megakaryocytic colonies which associated with PDPN+ stromal cells at periarteriolar space in the CLEC-2 WT and cKO BM. CD41+ nucleated cells and colonies localizing within 10 μm distance from PDPN+ stromal cells were defined as the megakaryocytes or immature megakaryocytic colonies associated with arteriole. Data were obtained using frozen BM sections from 3 individual CLEC-2 WT or CLEC-2 cKO mice. Scale bars, 100 μm for A and B; 50 μm for C, D, and F; 10 μm for E and magnified image in panel F. A, arteriole; B, bone; CV, central vein; S, sinusoid.
Characterization of BM PDPN-expressing stromal cells as BM FRC-like cells
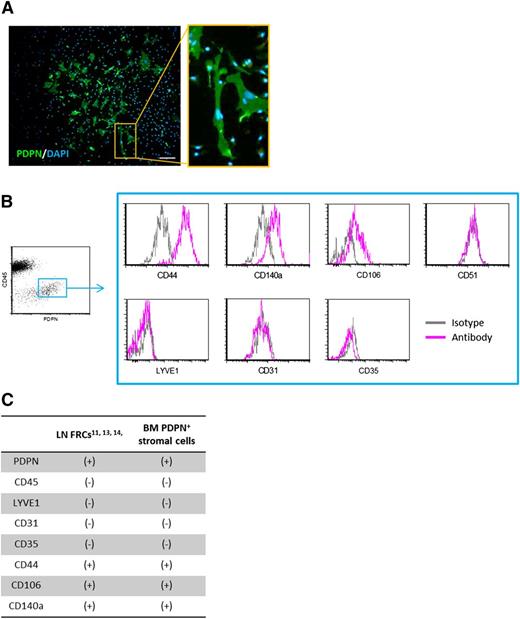
To further characterize the BM PDPN+ stromal cells, we separated and cultured BM adhesive stromal cells and found that they displayed fibroblastic morphology (Figure 3A). In general, vascular pericytes lack PDPN expression. However, FRCs that surround high endothelial venules in LNs are PDPN+ fibroblastic stromal cells.11-14 Because BM arteriolar pericytes, which appear to support megakaryocyte expansion, resemble LN FRCs in terms of morphology and PDPN expression, we compared the expression of several surface antigens between these 2 cell types. Immunophenotyping revealed that the BM PDPN+ stromal cells had a surface antigen pattern quite similar to that of LN FRCs (Figure 3B-C; supplemental Figure 3A). In addition, BM PDPN+ stromal cells were found to be negative for the osteoblastic lineage markers osteocalcin and N-cadherin (supplemental Figure 3B-C), indicating that PDPN+ stromal cells were distinct from other endosteal niche cells, such as spindle-shaped N-cadherin+CD45− osteoblastic cells.30 chemokine (C-X-C motif) ligand 12 (CXCL12)-abundant reticular (CAR) cells are widely distributed among sinusoids in the BM.31 CAR cells are niche cells that serve not only to maintain hematopoietic cells, but also to regulate erythrocyte and B-cell differentiation.32 We next investigated whether BM PDPN+ stromal cells were identical to CAR cells by evaluating the expression of CD51, a surface marker of CAR cells.32 However, fluorescence-activated cell sorter (FACS) analysis showed that BM PDPN+ stromal cells were CD51− (Figure 3B). From these observations, we tentatively termed these BM PDPN+ periarteriolar stromal cells as “BM FRC-like cells” based on their morphology and surface marker expression. To the best of our knowledge, this is the first report to suggest that arteriolar pericytes are PDPN+ in BM.
Characterization of BM periarteriolar PDPN-expressing stromal cells. (A) Representative ICC image of cultured PDPN+ BM stromal cells. Scale bar, 50 μm. (B) FACS immunophenotyping results of BM PDPN+ stromal cells. Cultured BM PDPN+ stromal cells are stained with LYVE1 (positive marker of LECs), CD31 (positive marker of vascular endothelial cells), CD35 (negative marker of LN FRCs), CD51 (positive marker of CAR cells), CD44, CD140a, and CD106 (positive marker of LN FRCs). The FACS scattergram and histograms show representative data from 3 independent experiments. (C) Immunophenotypic pattern of LN FRCs and BM PDPN+ stromal cells.
Characterization of BM periarteriolar PDPN-expressing stromal cells. (A) Representative ICC image of cultured PDPN+ BM stromal cells. Scale bar, 50 μm. (B) FACS immunophenotyping results of BM PDPN+ stromal cells. Cultured BM PDPN+ stromal cells are stained with LYVE1 (positive marker of LECs), CD31 (positive marker of vascular endothelial cells), CD35 (negative marker of LN FRCs), CD51 (positive marker of CAR cells), CD44, CD140a, and CD106 (positive marker of LN FRCs). The FACS scattergram and histograms show representative data from 3 independent experiments. (C) Immunophenotypic pattern of LN FRCs and BM PDPN+ stromal cells.
BM FRC-like cells augment megakaryocyte expansion through CLEC-2/PDPN interaction
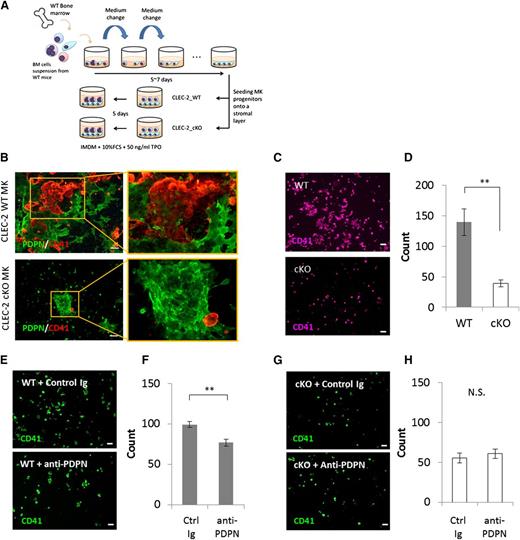
To mimic in vivo conditions where megakaryocyte lineages form expanded colonies at periarteriolar sites that associate with BM FRC-like cells, we cocultured megakaryocyte progenitors on a heterogeneous BM stromal cell layer in the presence of TPO (Figure 4A). The BM stromal cell layer contained ∼10% BM FRC-like cells, and all PDPN+ stromal cells were BM FRC-like cells (Figure 3A). After coculturing megakaryocyte progenitors with BM stromal cells, we found that CLEC-2 WT megakaryocyte colonies, but not CLEC-2 cKO megakaryocytes, associated with the BM FRC-like cells (Figure 4B). Quantitative analysis of ICC images revealed that the numbers of CLEC-2 cKO megakaryocytes were significantly reduced as compared with CLEC-2 WT megakaryocytes during coculture with BM stromal cells (Figure 4C-D). These results were consistent with the results of our colony-formation assay (Figure 1E). To confirm that this expansion was dependent on the CLEC-2/PDPN axis, we blocked the CLEC-2/PDPN interaction using a PDPN-neutralizing monoclonal antibody. As expected, abrogation of CELC-2/PDPN binding significantly suppressed the expansion of WT megakaryocyte lineages (Figure 4E-F), but had no effect on CLEC-2 cKO megakaryocytes (Figure 4G-H). It has been known that CLEC-2 is released from activated platelet as a soluble form by shedding.33 Therefore, we also investigated the possibility that soluble CLEC-2 is involved in a megakaryocyte expansion associated with BM FRC-like cells. We compared the megakaryocyte expansion in coculture with BM FRC-like cells in the presence or absence of GM6001 and GM6001. The GM6001 compound is a broad-range inhibitor of matrix metalloprotease that blocks membrane protein shedding. The GM6001 compound did not affect a megakaryocyte expansion associated with BM FRC-like cells (supplemental Figure 4A), suggesting that shed soluble CLEC-2 was not involved in the expansion. These results suggested that BM FRC-like cells stimulate megakaryocyte expansion via interactions between PDPN and CLEC-2 expressed on the respective cell surfaces.
BM FRC-like cells positively regulate megakaryocyte expansion via the CLEC-2/PDPN interaction. (A) Scheme of coculture of megakaryocyte progenitors within BM stromal layer containing BM FRC-like cells. (B) Representative ICC image of coculture of CLEC-2 WT megakaryocytes (top) or cKO megakaryocytes (bottom) with BM stromal cells. BM FRC-like cells were detected as PDPN+ cells (green). Megakaryocyte lineages were detected as CD41+ cells (red). (C-D) Expansion level of CLEC-2 cKO megakaryocytes was reduced as compared with that of CLEC-2 WT megakaryocytes in coculture with BM FRC-like cells. (E-H) Treatment with a PDPN blocking antibody (anti-PDPN, clone: 8F11, 15 μg/mL) suppressed megakaryocyte expansion of CLEC-2 WT (E-F), but not cKO cells (G-H) in coculture with BM FRC-like cells. Representative ICC images of CD41+ megakaryocytes in coculture with BM FRC-like cells (C,E,G), and quantitative absolute numbers of CD41+ megakaryocytes in fields, n = 4 per group (D,F,H). Scale bar, 100 μm for panels B, C, E, and G. The error bars represent SEMs. *P < .05, **P < .01. MK, megakaryocyte; N.S., a nonsignificant difference.
BM FRC-like cells positively regulate megakaryocyte expansion via the CLEC-2/PDPN interaction. (A) Scheme of coculture of megakaryocyte progenitors within BM stromal layer containing BM FRC-like cells. (B) Representative ICC image of coculture of CLEC-2 WT megakaryocytes (top) or cKO megakaryocytes (bottom) with BM stromal cells. BM FRC-like cells were detected as PDPN+ cells (green). Megakaryocyte lineages were detected as CD41+ cells (red). (C-D) Expansion level of CLEC-2 cKO megakaryocytes was reduced as compared with that of CLEC-2 WT megakaryocytes in coculture with BM FRC-like cells. (E-H) Treatment with a PDPN blocking antibody (anti-PDPN, clone: 8F11, 15 μg/mL) suppressed megakaryocyte expansion of CLEC-2 WT (E-F), but not cKO cells (G-H) in coculture with BM FRC-like cells. Representative ICC images of CD41+ megakaryocytes in coculture with BM FRC-like cells (C,E,G), and quantitative absolute numbers of CD41+ megakaryocytes in fields, n = 4 per group (D,F,H). Scale bar, 100 μm for panels B, C, E, and G. The error bars represent SEMs. *P < .05, **P < .01. MK, megakaryocyte; N.S., a nonsignificant difference.
PDPN binding to CLEC-2 augmented Akt phosphorylation in megakaryocytes
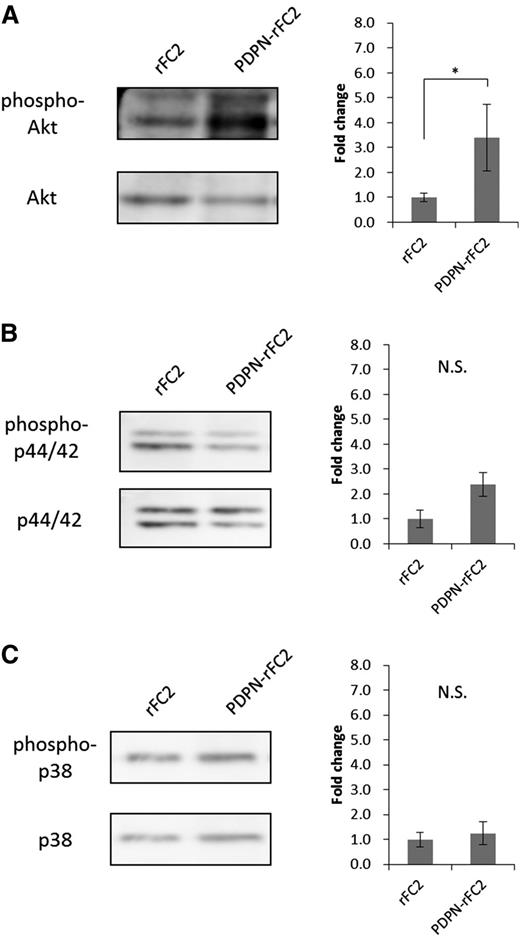
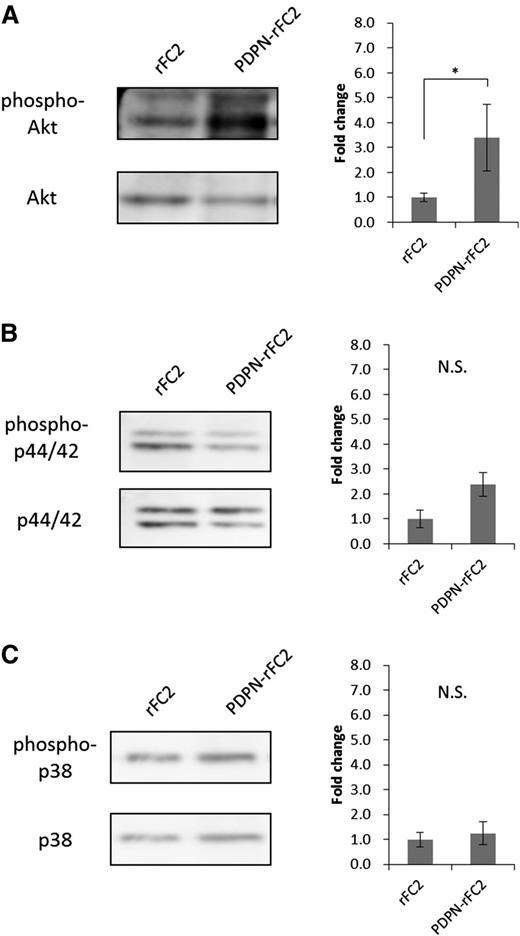
To investigate the mechanism by which the CLEC-2/PDPN interaction promotes megakaryocyte expansion, we analyzed the phosphorylation of signaling molecules in megakaryocytes stimulated with PDPN-rFC2. Moroi and Watson34 and Manne et al35 reported that CLEC-2 activation induced Akt, p44/42 (extracellular signal-regulated kinase 1/2), and p38 phosphorylation in platelets. Thus, we analyzed the phosphorylation levels of these signaling molecules in PDPN-rFC2–stimulated megakaryocytes. In the stimulated megakaryocytes, Akt phosphorylation levels significantly increased (Figure 5A), whereas those of p44/42 and p38 were not significantly altered (Figure 5B-C). These results suggested that PDPN binding to CLEC-2 positively regulates megakaryocyte expansion through the Akt pathway.
Akt phosphorylation levels increased in PDPN-stimulated megakaryocytes. Phosphorylation levels of Akt (A), p44/42 (B), and p38 (C) were examined by western blotting. The left images show representative bands for each target protein, and the results are quantified in the right graphs (n = 3 per group). The error bars reflect the SEMs. *P < .05. N.S., a nonsignificant difference.
Akt phosphorylation levels increased in PDPN-stimulated megakaryocytes. Phosphorylation levels of Akt (A), p44/42 (B), and p38 (C) were examined by western blotting. The left images show representative bands for each target protein, and the results are quantified in the right graphs (n = 3 per group). The error bars reflect the SEMs. *P < .05. N.S., a nonsignificant difference.
CLEC-2–stimulated BM FRC-like cells secrete soluble factors to promote PPF
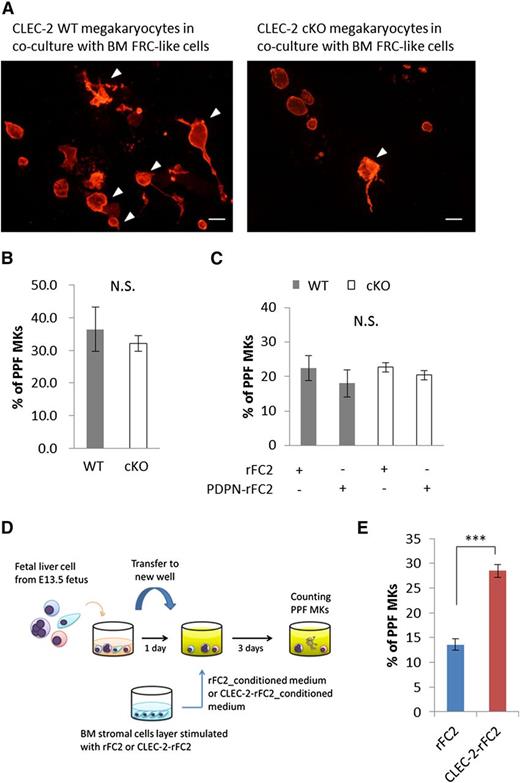
With regard to megakaryocyte maturation, we also found that a number of CLEC-2 WT megakaryocytes displayed proplatelet-like morphologies, with enlarged cytoplasm and a protrusion of pseudopods observed in cocultures with BM FRC-like cells; however, the proplatelet formation of CLEC-2 cKO megakaryocytes was reduced (Figure 6A). We therefore investigated the effect of CLEC-2 for PPF using fetal liver PPF assays.27 Notably, when megakaryocytes were cultured in the absence of BM FRC-like cells, the ratio of PPF in WT megakaryocytes to that in CLEC-2 cKO megakaryocytes was not significantly different (Figure 6B), suggesting that CLEC-2 deletion did not affect autonomous PPF. Moreover, the addition of PDPN-rFC2 into the culture medium also failed to promote PPF in either the CLEC-2 WT or cKO megakaryocytes (Figure 6C), suggesting that the CLEC-2/PDPN interaction does not directly facilitate PPF. We therefore hypothesized that the CLEC-2/PDPN axis may stimulate BM FRC-like cells to secrete unknown factors that facilitate PPF. To test this hypothesis, we used conditioned medium in our fetal liver PPF assays (Figure 6D) and found that CLEC-2–rFC2-conditioned medium significantly enhanced PPF when compared with rFC2-conditioned control medium (Figure 6E). This finding suggests that CLEC-2–rFC2-stimulated BM FRC-like cells secrete 1 or more PPF-promoting factors.
PPF by CLEC-2 WT megakaryocytes is facilitated by coculture with BM FRC-like cells. (A) Representative ICC images of proplatelet-forming megakaryocytes in coculture with BM FRC-like cells. CLEC-2 WT megakaryocytes (left panel) and CLEC-2 cKO megakaryocytes (right panel) were cocultured with BM FRC-like cells for 5 days. Megakaryocytes were stained with CD41 (red). Arrowheads indicate the proplatelet-forming megakaryocytes. Scale bar, 50 μm. (B-C) Quantification of PPF megakaryocytes in a fetal liver PPF model, with or without recombinant PDPN (PDPN-rFC2) treatment. (B) Cell-autonomous PPF in CLEC-2 WT (n = 3) and cKO megakaryocytes (n = 5). (C) Stimulated PPF with PDPN-rFC2 (20 μg/mL) in CLEC-2 WT and cKO megakaryocytes. CLEC-2 WT with rFC2, n = 3; CLEC-2 WT with PDPN, n = 3; CLEC-2 cKO with rFC2, n = 6; CLEC-2 cKO with PDPN, n = 7. (D) Scheme of the conditioned medium used in the fetal liver PPF assays. (E) Quantification of PPF with rFC2-conditioned medium or CLEC-2–conditioned medium, n = 6 per group. The error bars represent SEMs. ***P < .001. N.S., a nonsignificant difference.
PPF by CLEC-2 WT megakaryocytes is facilitated by coculture with BM FRC-like cells. (A) Representative ICC images of proplatelet-forming megakaryocytes in coculture with BM FRC-like cells. CLEC-2 WT megakaryocytes (left panel) and CLEC-2 cKO megakaryocytes (right panel) were cocultured with BM FRC-like cells for 5 days. Megakaryocytes were stained with CD41 (red). Arrowheads indicate the proplatelet-forming megakaryocytes. Scale bar, 50 μm. (B-C) Quantification of PPF megakaryocytes in a fetal liver PPF model, with or without recombinant PDPN (PDPN-rFC2) treatment. (B) Cell-autonomous PPF in CLEC-2 WT (n = 3) and cKO megakaryocytes (n = 5). (C) Stimulated PPF with PDPN-rFC2 (20 μg/mL) in CLEC-2 WT and cKO megakaryocytes. CLEC-2 WT with rFC2, n = 3; CLEC-2 WT with PDPN, n = 3; CLEC-2 cKO with rFC2, n = 6; CLEC-2 cKO with PDPN, n = 7. (D) Scheme of the conditioned medium used in the fetal liver PPF assays. (E) Quantification of PPF with rFC2-conditioned medium or CLEC-2–conditioned medium, n = 6 per group. The error bars represent SEMs. ***P < .001. N.S., a nonsignificant difference.
CCL5 (RANTES) is the PPF-promoting factor secreted from BM FRC-like cells
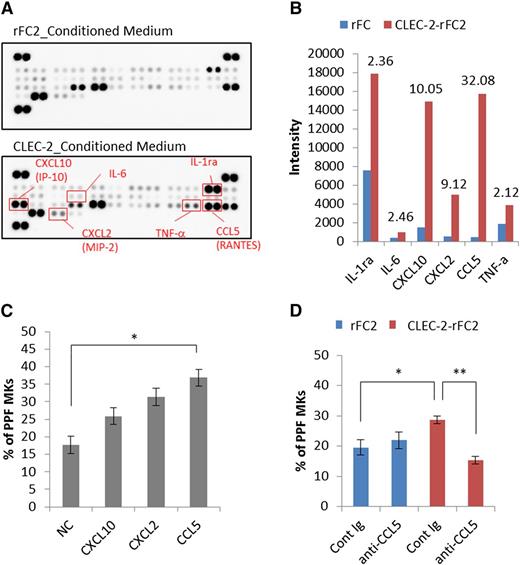
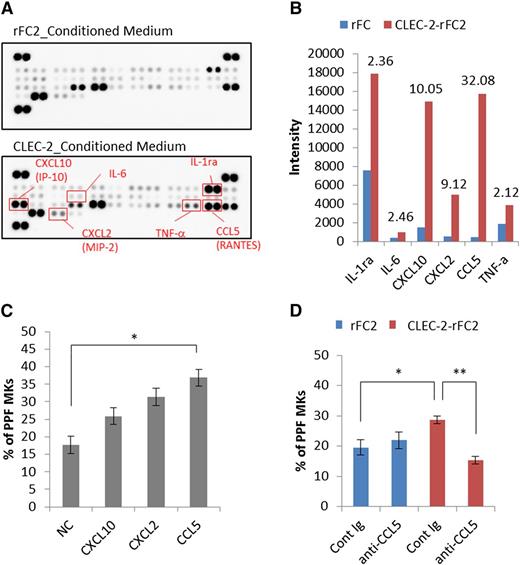
To identify PPF-promoting factors, we performed cytokine/chemokine arrays. Among the 40 proteins analyzed, the levels of CXCL10 (interferon-γ–inducible protein 10 [IP-10]), CXCL2 (macrophage inflammatory protein-2 [MIP-2]), and CCL5 (RANTES) increased more than fivefold in CLEC-2–rFC2-conditioned medium, compared with rFC2-control medium (Figure 7A-B; supplemental Figure 5). To determine the effect of these factors on PPF, we tested the recombinant candidate chemokines individually in fetal liver PPF assays, which revealed that only CCL5 promoted a significant increase in PPF (Figure 7C). Moreover, CCL5 neutralizing antibody markedly suppressed this effect in cultures with CLEC-2–rFC2-conditioned medium (Figure 7D). However, CCL5 did not enhance megakaryocyte colony expansion (supplemental Figure 6), suggesting that CCL5 specifically promotes megakaryocyte differentiation. These data suggested that the binding of megakaryocyte CLEC-2 to PDPN present on BM FRC-like cells results in CCL5 secretion from BM FRC-like cells, which then potentiates PPF in megakaryocytes.
The CLEC-2/PDPN interaction induces BM FRC-like cells to secrete a PPF-facilitating factor, CCL5. Identification of PPF-facilitating factor in CLEC-2–conditioned medium (supernatant from CLEC-2–stimulated BM FRC-like cells). (A) Results obtained using the Proteome Profiler Mouse Cytokine Array Panel A. The top and bottom panels indicate the acquired array images from rFC2-conditioned medium and CLEC-2–rFC2-conditioned medium, respectively. (B) Spot intensity on the Proteome Profiler Mouse Cytokine Array Panel A with rFC2- and CLEC-2–rFC2-conditioned medium. This graph shows quantitative results of the images shown in panel A. Values above each bar indicate the fold-change of rFC2-conditioned medium (blue bar) relative to CLEC-2–conditioned medium (red bar). (C) PPF assay with recombinant CXCL10, CXCL2, and CCL5 (n = 6 per group). (D) CCL5-neutralizing antibody (AF478, 1.5 μg/mL final) suppressed PPF in culture with CLEC-2–conditioned medium (n = 6 per group). The error bars represent SEMs. *P < .05, **P < .01. Cont Ig, control immunoglobulin; IL-1ra, IL-1 receptor antagonist; NC, negative control; TNF-a, tumor necrosis factor-α.
The CLEC-2/PDPN interaction induces BM FRC-like cells to secrete a PPF-facilitating factor, CCL5. Identification of PPF-facilitating factor in CLEC-2–conditioned medium (supernatant from CLEC-2–stimulated BM FRC-like cells). (A) Results obtained using the Proteome Profiler Mouse Cytokine Array Panel A. The top and bottom panels indicate the acquired array images from rFC2-conditioned medium and CLEC-2–rFC2-conditioned medium, respectively. (B) Spot intensity on the Proteome Profiler Mouse Cytokine Array Panel A with rFC2- and CLEC-2–rFC2-conditioned medium. This graph shows quantitative results of the images shown in panel A. Values above each bar indicate the fold-change of rFC2-conditioned medium (blue bar) relative to CLEC-2–conditioned medium (red bar). (C) PPF assay with recombinant CXCL10, CXCL2, and CCL5 (n = 6 per group). (D) CCL5-neutralizing antibody (AF478, 1.5 μg/mL final) suppressed PPF in culture with CLEC-2–conditioned medium (n = 6 per group). The error bars represent SEMs. *P < .05, **P < .01. Cont Ig, control immunoglobulin; IL-1ra, IL-1 receptor antagonist; NC, negative control; TNF-a, tumor necrosis factor-α.
Discussion
In this study, we demonstrate that (1) BM FRC-like cells are periarteriolar stromal cells that express PDPN in the BM, (2) PDPN binding to CLEC-2 positively regulates megakaryocyte expansion, and (3) CLEC-2/PDPN binding stimulates BM FRC-like cells to secrete CCL5 to promote PPF in megakaryocytes. The CLEC-2/PDPN axis between megakaryocytes and BM FRC-like cells constitutes a reciprocal interaction that generates the megakaryopoietic microenvironment at periarteriolar sites in the BM (supplemental Figure 7). These data support our hypothesis that BM FRC-like cells provide a CLEC-2/PDPN-dependent niche that potentiates megakaryocyte expansion and CCL5-mediated PPF.
In megakaryopoiesis, phosphatidylinositol 3-kinase/Akt signaling promotes the proliferation of megakaryocyte progenitors,36,37 and p44/42 signaling regulates megakaryocytic differentiation.38 Interestingly, p38 does not appear to be involved in megakaryopoietic processes.39 In this study, we found that PDPN binding to CLEC-2 increases Akt phosphorylation in megakaryocytes (Figure 5). Because the PDPN interaction with CLEC-2 in megakaryocytes led to megakaryocyte proliferation, the Akt pathway appears to be related to megakaryocytic expansion. Next, we investigated the role of CCL5/chemokine (C-C motif) receptor 5 (CCR5)40,41 -related signaling in megakaryocyte differentiation. We evaluated the phosphorylation levels signaling molecules downstream of CCR5,42 including myosin light chain, cofilin, and p44/42. However, no significant alterations in the phosphorylation levels of these molecules were observed (supplemental Figure 8). Thus, there may be an unknown signaling pathway(s) downstream of CCL5/CCR5 involved in megakaryocyte maturation.
This study also represents the first report that PDPN signaling associated with CLEC-2 binding induces chemokine secretion (Figures 5-6). Although it has been reported previously that CLEC-2 binding to PDPN induces morphologic changes in LN FRCs by modulating actomyosin contractility,13,14 it has not been demonstrated that PDPN signaling associated with CLEC-2 binding induces soluble factor secretion. In LECs, the binding of CLEC-2 to PDPN induces PDPN clustering, which elicits downstream signaling to modulate ezrin/radixin/moesin proteins.43 By analogy, we assume that megakaryocyte CLEC-2–mediated clustering of PDPN on BM FRC-like cells elicits intracellular signaling in BM FRC-like cells, which results in CCL5 secretion.
It is well documented and widely accepted that megakaryocyte progenitor expansion mainly occurs in proliferative endosteal niches and it is assumed that the expanded megakaryocyte population migrates toward the sinusoidal vasculatures (vascular niches) following a CXCL12 (stromal cell-derived factor 1) chemoattractant gradient secreted from sinusoidal endothelial cells.6,44 At vascular niches, megakaryocytes produce platelets and release them into the blood circulation.44,45 Our present observations raise the possibility that BM arterioles partly contribute to the formation of both proliferative and maturational microenvironments for megakaryopoiesis (supplemental Figure 9), which may not require megakaryocyte migration toward different niches. The BM matrix is densely packed with sinusoids, many of which appear to be juxtaposed to arterioles.46 We observed that mature megakaryocytes interacted with both PDPN+ arterioles and PDPN− sinusoids (Figure 2F). Thus, although the migration of mature megakaryocytes to sinusoids may indeed be necessary, it is possible that the close proximity of arterioles and sinusoids allows megakaryocytes to remain stationary during cell expansion, maturation, and proplatelet release. However, this hypothesis should be further evaluated. To completely demonstrate a role for the CLEC-2/PDPN-dependent megakaryopoietic niche in the BM, it will be necessary to generate cKO mice podoplanin deleted specifically in BM FRC-like cells, which is currently under investigation in our laboratory.
In summary, we propose a novel BM periarteriolar megakaryocytic niche that provides a proliferative and maturational microenvironment for megakaryocytes. A reciprocal CLEC-2/PDPN interaction between megakaryocytes and BM FRC-like cells accelerates megakaryopoiesis, resulting from the direct promotion of megakaryocytic clonal expansion and indirect facilitation of megakaryocyte PPF. This concept contributes to the understanding of a complex regulatory process for platelet production in the BM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Dr Shinji Kunishima for his technical advice on performing the PPF assays. They are also grateful to Hisaichiro Nakazawa, Uina Fukuda, and Junko Nakagomi for their technical assistance.
This work was supported in part by a Grant-in-Aid for Japan Society for the Promotion of Science fellows (25⋅4623), Young Scientist (B) (26860723), and the Funding Program for Next Generation World-leading Researchers (NEXT Program) (LS052). This work was also supported in part by grants from the SENSHIN Medical Research Foundation.
Authorship
Contribution: S.T. performed the majority of experiments, designed the study, analyzed data, and wrote the manuscript; K.S.-I. designed the study, analyzed data, wrote the manuscript, and supervised the study; N.T., T. Shirai, and T. Sasaki performed the experiments and statistical analysis; M.O. and K.S. analyzed the data; Y.O. designed the study, analyzed data, wrote the manuscript, and supervised the study; and all authors discussed the results and contributed to manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.T. is Department of Pathophysiological Laboratory Sciences, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Correspondence: Katsue Suzuki-Inoue, Department of Clinical and Laboratory Medicine, Faculty of Medicine, University of Yamanashi, 1110 Shimokato, Chuo, Yamanashi 409-3898, Japan; e-mail: katsuei@yamanashi.ac.jp.