Key Points
STAT3 decoy conjugated to TLR9 ligand targets survival and immune checkpoint signaling in all acute myeloid leukemia cell compartments.
Serum-resistant CpG-STAT3dODN conjugates induce direct killing and/or immune-mediated eradication of AML, including stem/progenitor cells.
Abstract
Targeting oncogenic transcription factor signal transducer and activator of transcription 3 (STAT3) in acute myeloid leukemia (AML) can reduce blast survival and tumor immune evasion. Decoy oligodeoxynucleotides (dODNs), which comprise STAT3-specific DNA sequences are competitive inhibition of STAT3 transcriptional activity. To deliver STAT3dODN specifically to myeloid cells, we linked STAT3dODN to the Toll-like receptor 9 (TLR9) ligand, cytosine guanine dinucleotide (CpG). The CpG-STAT3dODN conjugates are quickly internalized by human and mouse TLR9+ immune cells (dendritic cells, B cells) and the majority of patients’ derived AML blasts, including leukemia stem/progenitor cells. Following uptake, CpG-STAT3dODNs are released from endosomes, and bind and sequester cytoplasmic STAT3, thereby inhibiting downstream gene expression in target cells. STAT3 inhibition in patients’ AML cells limits their immunosuppressive potential by reduced arginase expression, thereby partly restoring T-cell proliferation. Partly chemically modified CpG-STAT3dODNs have >60 hours serum half-life which allows for IV administration to leukemia-bearing mice (50% effective dose ∼ 2.5 mg/kg). Repeated administration of CpG-STAT3dODN resulted in regression of human MV4-11 AML in mice. The antitumor efficacy of this strategy is further enhanced in immunocompetent mice by combining direct leukemia-specific cytotoxicity with immunogenic effects of STAT3 blocking/TLR9 triggering. CpG-STAT3dODN effectively reduced Cbfb/MYH11/Mpl AML burden in various organs and eliminated leukemia stem/progenitor cells, mainly through CD8/CD4 T-cell–mediated immune responses. In contrast, small-molecule Janus kinase 2/STAT3 inhibitor failed to reproduce therapeutic effects of cell-selective CpG-STAT3dODN strategy. These results demonstrate therapeutic potential of CpG-STAT3dODN inhibitors with broad implications for treatement of AML and potentially other hematologic malignancies.
Introduction
Growing evidence suggests that successful reversal of tumor immune evasion requires strategies combining potent immunostimulation with the inhibition of immune checkpoints.1,2 Similar to those with other hematologic malignancies, acute myeloid leukemia (AML) patients show a high frequency of signal transducer and activator of transcription 3 (STAT3) activation in leukemic blasts, which correlates with worse outcome.3-7 STAT3 plays a role in promoting AML cell proliferation and survival while preventing leukemic cell differentiation.8-11 In addition, as recently shown, STAT3 activation in AML cells and in leukemia-associated myeloid cell populations plays a critical role in inducing and sustaining tumor immune tolerance.12 STAT3 is an attractive but challenging target for cancer therapy, as pharmacologic inhibition of nonenzymatic proteins proved difficult.5,13 Synthetic oligonucleotides provide an opportunity for targeting STAT3 at the level of gene expression or transcriptional activity.14 Decoy oligodeoxynucleotides (dODNs) comprise the consensus DNA-binding sequences for specific transcription factors and when delivered intracellularly act as competitive inhibitors. Grandis and colleagues pioneered the use of STAT3dODN for therapy of head and neck cancers, selected due to the optimal internalization rate of the unformulated STAT3dODN by these cancer cells.15,16 The STAT3dODN demonstrated good safety profiles and target inhibition in recent clinical testing (phase 0) using intratumoral injections.16 The limitation remained local administration and the limited ability of naked STAT3dODN to penetrate immune cells in the tumor microenvironment constraining potential antiangiogenic and immunostimulatory effects.13
Our previous studies demonstrated that cytosine guanine dinucleotide (CpG) ODNs, ligands for intracellular innate immune receptor Toll-like receptor 9 (TLR9), can be efficiently used for targeted small interfering RNA (siRNA) delivery to mouse and human TLR9-positive cells.12,17,18 To overcome the sensitivity of siRNA to degradation in human serum, we currently report the design of chemically modified CpG-STAT3dODN conjugates suitable for IV administration against human AML.
Methods
Cells
Normal peripheral blood mononuclear cells (PBMCs) were derived from anonymous healthy donors (institutional review board [IRB] no. 13378), whereas patients’ derived leukemic blasts were from the City of Hope (COH) repository (IRB no. 3162) or kindly provided by Drs Danet-Desnoyers and DosSantos (IRB no. 703185; University of Pennsylvania). Cell viability was >90% as confirmed using flow cytometry. Sample acquisition was approved by the respective institutional review board in accordance with the Declaration of Helsinki. Human myeloid/plasmacytoid dendritic cells (mDCs/pDCs) were cultured from PBMC-derived monocytes as described.18 To generate MV4-11ch/luc cells, parental MV4-11 cells were transduced with luciferase/mCherry using a lentiviral vector provided by Dr A. Kung (Columbia University).19 Generation of the Cbfb-MYH11/Mpl+(CMM) model was described before.12,20 Mouse RAW264.7 macrophages were purchased from ATCC; human KG1a and MV4-11 leukemia cell lines were originally obtained from the laboratory of Dr D. Tenen (Beth Israel Deaconess Medical Center, Boston, MA).
Mice
All animal experiments were performed in accordance with established institutional guidance and approved protocols from the institutional animal care and use committee (COH). Six- and 8-week-old C57BL/6 mice were purchased from The Jackson Laboratory. The NOD/SCID/IL-2RγKO (NSG) colony from the National Cancer Institute (NCI; Frederick, MD) was maintained at COH. TLR9KO mice were originally from S. Akira (Osaka University). NSG or C57BL/6 mice were injected into the lateral tail vein with 0.5 × 106 MV4-11ch/luc or CMM cells in phosphate-buffered saline (PBS), respectively. The percentage of circulating hCD45+ MV4-11 or c-Kit+/green fluorescent protein–positive (GFP+) CMM cells was monitored cytofluorimetrically and after exceeding 1% to 5%, NSG and C57BL/6 mice were injected IV using CpG-dODNs daily or every other day, respectively. MV4-11ch/luc tumor progression was monitored using bioluminescent imaging on IVIS 100 (Xenogen).
CpG-STAT3dODN design and synthesis
The CpG-dODNs were synthesized in the DNA/RNA Synthesis Core (COH) by linking CpG-D19 or CpG-1668 ODNs to STAT3 decoy similarly as described.17 The resulting ODN conjugates are shown below (x indicates a single C3 unit; asterisks indicate phosphothioation sites):
CpG(D19)-STAT3dODN
5′ G*G*TGCATCGATGCAGG*G*G*G*G-xxxxx-C*A*T*TTCCCGTAAATC-xxxx-GATTTACGGGAA*A*T*G-xxxxx 3′
GpC(D19)-STAT3dODN
5′ G*G*TGCATGCATGCAGG*G*G*G*G-xxxxx-C*A*T*TTCCCGTAAATC-xxxx-GATTTACGGGAA*A*T*G-xxxxx 3′
CpG(D19)-scrambled ODN
5′ G*G*TGCATCGATGCAGG*G*G*G*G-xxxxx- A*C*T*CTTGCCAATTAC-xxxx-GTAATTGGCAAG*A*G*T-xxxxx 3′
CpG(1668)-STAT3dODN
5′ T*C*C*A*T*G*A*C*G*T*T*C*C*T*G*A*T*G*C*T-xxxxx-C*A*T*TTCCCGTAAATC-xxxx-GATTTACGGGAA*A*T*G-xxxxx 3′
CpG(1668)-point-mutated STAT3dODN
5′ T*C*C*A*T*G*A*C*G*T*T*C*C*T*G*A*T*G*C*T-xxxxx-C*A*T*TTCCCTTAAATC-xxxx-GATTTAAGGGAA*A*T*G-xxxxx 3′
CpG(1668)-scrambled ODN
5′ T*C*C*A*T*G*A*C*G*T*T*C*C*T*G*A*T*G*C*T-xxxxx-A*C*T*CTTGCCAATTAC-xxxx-GTAATTGGCAAG*A*G*T-xxxxx 3′
STAT3dODN alone
5′ xxxxx-C*A*T*TTCCCGTAAATC-xxxx-GATTTACGGGAA*A*T*G-xxxxx 3′
For uptake studies, ODNs were labeled using Alexa 488, fluorescein isothiocyanate (FITC), or Cy3.
Confocal microscopy
RAW264.7 cells were cultured on coverslips in 24-well plates, fixed in 2% paraformaldehyde (EMS), permeabilized with 0.1% Triton X-100 (Sigma-Aldrich), and stained using primary anti-tyrosine-phosphorylated STAT3 (pSTAT3; Cell Signaling) and Alexa 488–coupled secondary antibodies (Life Technologies). Slides mounted in Vectashield Hard-Set medium (Vector Laboratories) were visualized on an LSM510-Axiovert inverted confocal microscope (Zeiss) and analyzed using LSM ImageBrowser (version 4.2.0.121; Zeiss). The proximity ligation assays were performed using FITC- and pSTAT3-specific antibodies as reported (supplemental Methods, available on the Blood Web site).21
EMSAs and protein assays
Electrophoretic mobility shift assays (EMSAs) to detect transcription factor DNA-binding activity were performed as described previously.17 Briefly, nuclear extracts (10 µg) were incubated with 32P-labeled oligonucleotide probes specific for: STAT3 (high-affinity sis-inducible element [hSIE]), STAT5 (mammary gland factor element [MGF]),22 or nuclear factor–κB (NF-κB).23 Protein-DNA complexes were resolved on 5% nondenaturing polyacrylamide gel electrophoresis and detected by autoradiography. For supershifting, nuclear extracts were preincubated for 30 minutes with anti-STAT3 antibody (Santa Cruz Biotechnology). Western blot to detect STAT3, pSTAT3, BCL-XL, and β-actin expression was described before.18 Arginase-1 enzymatic activity in supernatants collected from cultured primary patients’ derived AML samples were measured using the QuantiChrom Assay (BioAssay Systems) as described.24
Luciferase reporter assay
KG1a and MV4-11 cells were treated with indicated concentrations of CpG-STAT3dODN, CpG-scrambled ODN (scrODN), or STAT3dODN for 18 hours, then washed and cotransfected with α2-macroglobulin promoter (pGL3/α2M-luc) luciferase-reporter plasmid or control pGL3-luc plasmid plus Renilla luciferase (pRL-TK) using Lipofectamine 2000 (Life Technologies). Cells were harvested 30 hours after transfection, and analyzed using the Dual-Luciferase Reporter Assay (Promega) following the manufacturer’s protocol.
Flow cytometry and T-cell assays
Single-cell suspensions were prepared by mechanic tissue disruption.25 The AML percentages were determined by GFP and c-Kit expression. For extracellular staining, fluorochrome-labeled antibodies to major histocompatibility complex (MHC) class II, CD3, CD4, CD8, CD11b, CD40, CD80, CD86, or Gr1 after anti-FcγIII/IIRBlock were used (eBioscience). Human immune cells and PBMCs were analyzed using the following antibodies: HLA-DR, CD1c, CD3, CD14, CD19, CD40, CD80, CD86, CD303 (eBioscience). For intracellular staining, cells were fixed/permeabilized and immunostained for TLR9 (eBioscience), STAT3P, FoxP3 (eBioscience), or Arginase-1 (R&D Systems) as described.12,24 Fluorescence data were analyzed on BD Fortessa and an AccuriC6 Flow Cytometer (BD) using FlowJo software (TreeStar).
To assess T-cell proliferation, CD3+ T cells, isolated from healthy subjects’ PBMCs using antibody-based/magnetic bead enrichment (Stemcell Technologies) were labeled using carboxyfluorescein succinimidyl ester (CFSE; Life Technologies). CFSE-labeled cells were incubated with CD3/CD28 Dynabeads (Life Technologies) for 3 days with or without AML cells and analyzed for T-cell proliferation using an AccuriC6 flow cytometer (BD).
Statistics
The unpaired t test was used to calculate the 2-tailed P value to estimate statistical significance of differences between 2 experimental groups. One-way analysis of variance plus Bonferroni posttest were applied to assess the statistical significance of differences between multiple treatment groups. The relationship between 2 groups was assessed using correlation and linear regression. The P values and r2 are indicated in the figures with asterisks: ***P < .001; **P < .01; *P < .05. Data were analyzed using Prism software (version 6.03; GraphPad).
Results
Design and cell-selective intracellular uptake of CpG-STAT3dODN
To generate myeloid cell-specific STAT3 inhibitors suitable for systemic delivery and able to bypass serum endonucleases, we conjugated a STAT3 decoy (STAT3dODN) to CpG-D19 or CpG-1668 ODNs which are optimized for stimulation of either human or mouse immune cells, respectively.17,18 For the conjugate design, we used the consensus STAT3 DNA-binding sequence26 and converted it into hairpin ODN by adding synthetic carbon linkers to both of the decoy strands (Figure 1A). The resulting conjugate was partly phosphorothioated within the CpG part and within the STAT3dODN hairpin stem in order to resist serum nucleases. Both D19 and 1668 types of the chemically modified CpG-STAT3dODNs had half-lives exceeding 2 days when incubated in human (T1/2 = 63 hours and 67 hours, respectively) or mouse sera (T1/2 = 87 hours and 90 hours, respectively) (supplemental Figure 1).
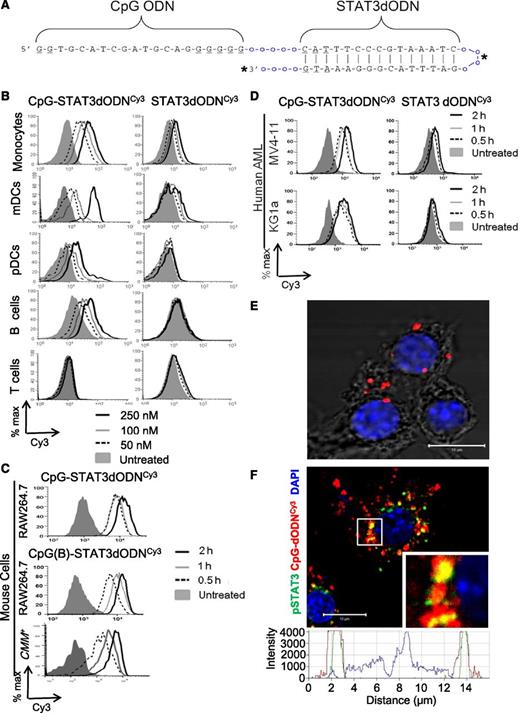
Design and cell-selective intracellular uptake of the CpG-STAT3 decoy oligonucleotide. (A) Predicted hairpin structure of the CpG-STAT3dODN consisting of CpG-D19 ODN linked to the double-stranded STAT3 decoy. Underlined are phosphothioation sites in the ODN backbone; o = single unit of the C3 carbon chain (CH2)3; asterisks (*) indicate fluorochrome conjugation sites (Cy3, 3′ end; FITC, in the linker). (B-D) Dose- and time-dependent internalization of CpG-STAT3dODN by target immune and leukemic cells without any transfection reagents. CpG(D19)-STAT3dODN or unconjugated STAT3dODN molecules were Cy3-labeled to detect their intracellular uptake by target cells using flow cytometry. (B) Human immune cells were incubated with indicated concentrations of CpG(D19)-STAT3dODNCy3 or STAT3dODNCy3 for 4 hours. The oligonucleotide uptake by CD14+ monocytes, CD1c+ (BDCA1+) mDCs, CD303+(BDCA2+) pDCs, CD19+ B cells, and CD3+ T cells was measured using flow cytometry. Similar results were obtained from 3 independent experiments. (C) Mouse RAW264.7 macrophages and CMM+ (Cbfb/MYH11/Mpl+) leukemia cells rapidly internalize CpG(D19)- and CpG(1668)-STAT3dODN conjugates. Cells were incubated with 250 nM Cy3-labeled conjugates for indicated times. (D) Cultured human AML cells (MV4-11, KG1a) quickly internalize CpG(D19)-STAT3dODN (500 nM) but not unconjugated STAT3 decoy. (E-F) CpG-STAT3dODN colocalizes and directly interacts with cytoplasmic STAT3 after internalization into target cells. (E) Direct interaction between CpG(D19)-STAT3dODNFITC and activated STAT3 as verified using FITC- and pSTAT3-specific antibodies and proximity ligation assay. RAW264.7 macrophages were incubated with 500 nM CpG(D19)-STAT3dODNFITC for 1 hour, fixed, and permeabilized. The interaction between the oligonucleotide and pSTAT3 using in situ proximity ligation assay (PLA) with FITC- and pSTAT3-specific antibodies labeled with PLA probes. The close proximity of both molecules is indicated by cyclic polymerase reaction producing red fluorescent spots in the cytoplasm. Shown is the image representative for 1 of 3 independent experiments with similar results; scale bar, 10 μm. (F) The intracellular localization of the FITC-labeled oligonucleotide and activated STAT3 was assessed using confocal microscopy after staining with pSTAT3-specific antibodies; inlay, the enlargement of the perinuclear area; bottom, overlay of signal intensities for fluorescent channels across the cell of interest as indicated by the dotted line: green, CpG-dODNFITC; red, pSTAT3; blue, nuclear staining with DAPI. Shown are representative images from 1 of 3 independent experiments with similar results; scale bar = 10 µm. % max, percentage of maximum; DAPI, 4,6 diamidino-2-phenylindole.
Design and cell-selective intracellular uptake of the CpG-STAT3 decoy oligonucleotide. (A) Predicted hairpin structure of the CpG-STAT3dODN consisting of CpG-D19 ODN linked to the double-stranded STAT3 decoy. Underlined are phosphothioation sites in the ODN backbone; o = single unit of the C3 carbon chain (CH2)3; asterisks (*) indicate fluorochrome conjugation sites (Cy3, 3′ end; FITC, in the linker). (B-D) Dose- and time-dependent internalization of CpG-STAT3dODN by target immune and leukemic cells without any transfection reagents. CpG(D19)-STAT3dODN or unconjugated STAT3dODN molecules were Cy3-labeled to detect their intracellular uptake by target cells using flow cytometry. (B) Human immune cells were incubated with indicated concentrations of CpG(D19)-STAT3dODNCy3 or STAT3dODNCy3 for 4 hours. The oligonucleotide uptake by CD14+ monocytes, CD1c+ (BDCA1+) mDCs, CD303+(BDCA2+) pDCs, CD19+ B cells, and CD3+ T cells was measured using flow cytometry. Similar results were obtained from 3 independent experiments. (C) Mouse RAW264.7 macrophages and CMM+ (Cbfb/MYH11/Mpl+) leukemia cells rapidly internalize CpG(D19)- and CpG(1668)-STAT3dODN conjugates. Cells were incubated with 250 nM Cy3-labeled conjugates for indicated times. (D) Cultured human AML cells (MV4-11, KG1a) quickly internalize CpG(D19)-STAT3dODN (500 nM) but not unconjugated STAT3 decoy. (E-F) CpG-STAT3dODN colocalizes and directly interacts with cytoplasmic STAT3 after internalization into target cells. (E) Direct interaction between CpG(D19)-STAT3dODNFITC and activated STAT3 as verified using FITC- and pSTAT3-specific antibodies and proximity ligation assay. RAW264.7 macrophages were incubated with 500 nM CpG(D19)-STAT3dODNFITC for 1 hour, fixed, and permeabilized. The interaction between the oligonucleotide and pSTAT3 using in situ proximity ligation assay (PLA) with FITC- and pSTAT3-specific antibodies labeled with PLA probes. The close proximity of both molecules is indicated by cyclic polymerase reaction producing red fluorescent spots in the cytoplasm. Shown is the image representative for 1 of 3 independent experiments with similar results; scale bar, 10 μm. (F) The intracellular localization of the FITC-labeled oligonucleotide and activated STAT3 was assessed using confocal microscopy after staining with pSTAT3-specific antibodies; inlay, the enlargement of the perinuclear area; bottom, overlay of signal intensities for fluorescent channels across the cell of interest as indicated by the dotted line: green, CpG-dODNFITC; red, pSTAT3; blue, nuclear staining with DAPI. Shown are representative images from 1 of 3 independent experiments with similar results; scale bar = 10 µm. % max, percentage of maximum; DAPI, 4,6 diamidino-2-phenylindole.
Next, we assessed immune cell-specific uptake of Cy3 fluorochrome-labeled CpG-STAT3dODNs and the unconjugated STAT3dODN using flow cytometry (Figure 1B). After 4-hour incubation with human PBMCs, we observed that CpG(D19)-STAT3dODNCy3 was dose-dependently internalized by CD14+ monocytes, cultured CD1c+ mDCs, CD303+ pDCs, and CD19+ B cells but not CD3+ T cells. In contrast, all tested immune cell populations failed to internalize the STAT3dODN alone at concentrations up to 250 nM (Figure 1B). Similar myeloid- and B-cell specific uptake was also observed for the CpG(1668)-STAT3dODN using primary mouse immune cells (supplemental Figure 2). Mouse myeloid cells internalized both CpG-D19- and CpG-1668-STAT3dODN conjugates within 30 minutes of incubation (Figure 1C). Our previous studies demonstrated that in addition to nonmalignant myeloid cells, CpG conjugates can also target AML cells. As shown in Figure 1C-D, both mouse Cbfb/MHY11/Mpl+ (CMM) leukemia and human AML cells rapidly uptake CpG-STAT3dODN but not the unconjugated decoy after 2 hours. Similar to CpG-siRNA conjugates,21 CpG-STAT3dODN uptake by myeloid cells relies on scavenger receptor-mediated endocytosis as verified cytofluorimetrically (supplemental Figure 3A). Following the rapid internalization into early endosomes within 15 minutes (supplemental Figure 3B), the CpG-STAT3dODN is released into cytoplasm and at 30 minutes begins to directly interact with activated, pSTAT3, as shown using proximity ligation assay and confocal microscopy (Figure 1E; supplemental Figure 4A). After 48-hour incubation, CpG-STAT3dODN colocalized with most of pSTAT3 in the cytoplasm thereby interfering with STAT3 nuclear translocation as visualized using confocal microscopy (Figure 1F; supplemental Figure 4B). Thus, we anticipated that delivery of the CpG-STAT3 decoy conjugate will inhibit STAT3 activity in normal and malignant myeloid cells.
CpG-STAT3dODN inhibits transcriptional activity of STAT3 in myeloid cells
STAT3 decoys bind with high affinity to activated STAT3 dimers reducing their transcriptional activity.26-28 To verify whether a CpG-STAT3dODN conjugate exerts similar activity, we tested binding of STAT3 to its radiolabeled target DNA sequences using EMSA. As shown in Figure 2A, CpG-STAT3dODN abrogated interleukin-6 (IL-6)– and IL-10–induced STAT3 DNA binding in mouse splenocytes and also reduced constitutive STAT3 activity in KG1a AML cells. The control CpG ODN conjugates with inactivated, point-mutated (CpG-mutODN) or with scrambled (CpG-scrODN) decoy sequence showed opposite effects and upregulated STAT3 activity. This was likely a result of CpG/TLR9-induced autocrine expression of IL-6 and/or IL-10.29,30 Consistent with other reports,26,27 the STAT3 decoy sequence also reduces DNA binding of a closely related transcription factor, STAT1 (Figure 2A). Although STAT3 and STAT1 often have opposing biological effects on immune cells, both can have similar tumor-promoting effects in leukemia and other cancer cells.31-33 In contrast, CpG-STAT3dODN did not reduce DNA binding of STAT5 or NF-κB (p65/p50) transcription factors, thus confirming the overall specificity of CpG-STAT3dODN (Figure 2A right).
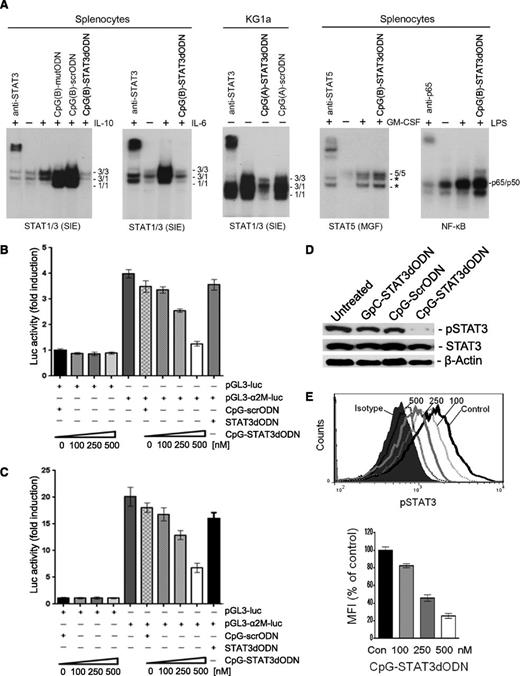
CpG-STAT3dODN inhibits STAT3 DNA binding, tyrosine phosphorylation, and transcriptional activity in target immune and leukemic cells in vitro. (A) CpG-STAT3dODN inhibits DNA binding of STAT3 and STAT1 but not other transcription factors (TFs). Mouse splenocytes or human KG1a leukemic cells were incubated with indicated CpG-STAT3dODN, control CpG-scrODN (scrambled decoy), or CpG-mutODN (point-mutated decoy) at 500 nM for 48 hours; CpG(A) = D19, CpG(B) = 1668. Cells were then stimulated as indicated or left untreated before isolation of nuclear extracts. The DNA-binding activity of various transcription factors was assessed in EMSA using radiolabeled probes specific for STAT1/STAT3, STAT5, and NF-κB; band identities were verified using specific antibodies; *, unspecific bands. Results represent at least 2 independent experiments with similar outcome. (B-C) CpG-STAT3dODN dose-dependently reduces transcriptional STAT3 activity. MV4-11 (B) and KG1a (C) AML cells were incubated for 18 hours with indicated doses of CpG-STAT3dODN or control CpG-scrODN and then transfected with STAT3-responsive α2M-promoter-luciferase reporter plasmid or empty vectors, together with Renilla-luciferase plasmid to control transfection efficiency. After 30 hours, the level of STAT3-induced transcriptional activity was assessed in cell lysates by measuring dual-luciferase activity in 2 independent experiments in triplicates; means ± standard error of the mean (SEM) (n = 3). (D-E) CpG-STAT3dODN reduces STAT3 activation but not protein levels in AML cells. (D) Levels of pSTAT3 and total STAT3 protein in human MV4-11 AML cells incubated for 48 hours with 500 nM CpG(D19)-STAT3dODN in comparison with CpG(D19)-scrODN or GpC-STAT3dODN used as negative control. Representative western blotting results showing pSTAT3 and STAT3 protein levels, using β-actin to control loading. (E) Dose-dependent inhibition of STAT3 phosphorylation by CpG-STAT3dODN. Mouse splenocytes were incubated with different concentrations of CpG(1668)-STAT3dODN or CpG(1668)-scrODN control for 48 hours, then stimulated for 20 minutes using IL-6 (20 ng/mL). pSTAT3 levels were assessed using intracellular staining with specific antibodies and flow cytometry. Representative histogram overlay (top) and averaged mean fluorescence intensities (MFIs) from triplicate samples (bottom); shown are means ± SEM (n = 3). GM-CSF, granulocyte macrophage colony-stimulating factor; LPS, lipopolysaccharide.
CpG-STAT3dODN inhibits STAT3 DNA binding, tyrosine phosphorylation, and transcriptional activity in target immune and leukemic cells in vitro. (A) CpG-STAT3dODN inhibits DNA binding of STAT3 and STAT1 but not other transcription factors (TFs). Mouse splenocytes or human KG1a leukemic cells were incubated with indicated CpG-STAT3dODN, control CpG-scrODN (scrambled decoy), or CpG-mutODN (point-mutated decoy) at 500 nM for 48 hours; CpG(A) = D19, CpG(B) = 1668. Cells were then stimulated as indicated or left untreated before isolation of nuclear extracts. The DNA-binding activity of various transcription factors was assessed in EMSA using radiolabeled probes specific for STAT1/STAT3, STAT5, and NF-κB; band identities were verified using specific antibodies; *, unspecific bands. Results represent at least 2 independent experiments with similar outcome. (B-C) CpG-STAT3dODN dose-dependently reduces transcriptional STAT3 activity. MV4-11 (B) and KG1a (C) AML cells were incubated for 18 hours with indicated doses of CpG-STAT3dODN or control CpG-scrODN and then transfected with STAT3-responsive α2M-promoter-luciferase reporter plasmid or empty vectors, together with Renilla-luciferase plasmid to control transfection efficiency. After 30 hours, the level of STAT3-induced transcriptional activity was assessed in cell lysates by measuring dual-luciferase activity in 2 independent experiments in triplicates; means ± standard error of the mean (SEM) (n = 3). (D-E) CpG-STAT3dODN reduces STAT3 activation but not protein levels in AML cells. (D) Levels of pSTAT3 and total STAT3 protein in human MV4-11 AML cells incubated for 48 hours with 500 nM CpG(D19)-STAT3dODN in comparison with CpG(D19)-scrODN or GpC-STAT3dODN used as negative control. Representative western blotting results showing pSTAT3 and STAT3 protein levels, using β-actin to control loading. (E) Dose-dependent inhibition of STAT3 phosphorylation by CpG-STAT3dODN. Mouse splenocytes were incubated with different concentrations of CpG(1668)-STAT3dODN or CpG(1668)-scrODN control for 48 hours, then stimulated for 20 minutes using IL-6 (20 ng/mL). pSTAT3 levels were assessed using intracellular staining with specific antibodies and flow cytometry. Representative histogram overlay (top) and averaged mean fluorescence intensities (MFIs) from triplicate samples (bottom); shown are means ± SEM (n = 3). GM-CSF, granulocyte macrophage colony-stimulating factor; LPS, lipopolysaccharide.
We further investigated whether reduced target DNA binding results in decreased transcriptional activity of STAT3 in leukemic cells. For this purpose, we used a short fragment of the α2-macroglobulin (α2-M) promoter which contains STAT3-binding sites and is highly sensitive to STAT3-mediated signaling.34 MV4-11 and KG1a AML cells were treated using CpG-STAT3dODN or control CpG-scrODN and unconjugated STAT3dODN for 18 hours before transfection with α2-M promoter luciferase (pGL3 α2-M-luc) or control (pGL3-luc) plasmids. CpG-STAT3dODN reduced α2-M promoter activity in a dose-dependent manner in both MV4-11 (Figure 2B) and KG1a (Figure 2C) cells with no detectable inhibitory effect of control CpG-scrODN or unconjugated STAT3dODN.
Previous studies indicated that small-molecule STAT3 inhibitors or decoys, which block STAT3 DNA binding, also eventually reduce STAT3 phosphorylation.25,35,36 Indeed, we found that CpG-STAT3dODN decreased pSTAT3 but not total STAT3 in MV4-11 leukemia cells after 48-hour incubation as measured by western blot (Figure 2D). Neither CpG-scrODN nor GpC-STAT3dODN, which lacks TLR9-targeting motif, affected STAT3 phosphorylation (Figure 2D). Similar dose-dependent inhibition of STAT3 phosphorylation was confirmed using flow cytometry in mouse splenocytes (Figure 2E).
Targeting AML cells using systemic administration of CpG-STAT3dODN
STAT3 is often activated in leukemic cells, thereby providing survival signaling.5 Consistent with this fact, we observed induction of cell death in human AML cells, including MV4-11, primary AML blasts, and mouse CMM leukemic cells treated in vitro using 500 nM CpG-STAT3dODN but not CpG-scrODN control (supplemental Figure 5). In contrast, we did not observe toxicities in human PBMCs and mouse hematopoietic stem cells within 3 days’ incubation (supplemental Figure 6). These results prompted us to test the feasibility of using this strategy in vivo. First, we evaluated whether IV injection of CpG-STAT3dODN could reach target AML cells in peripheral organs in mice. Mice with established MV4-11ch/luc xenografts were injected IV with 5 mg/kg of fluorescently labeled CpG-STAT3dODNCy3. The oligonucleotide biodistribution was measured using flow cytometry as a percentage of Cy3-positive MV4-11 cells in blood, bone marrow, or spleen at 3 or 18 hours later (Figure 3A). The CpG-STAT3dODNCy3 was detectable in the majority of splenic and over 30% of circulating and bone marrow–resident AML cells at 3 hours and decreasing thereafter. Next, we measured the effect of 3 repeated CpG-STAT3dODN injections on STAT3 activity in AML cells in the spleen. As shown in Figure 3B, IV injections of CpG-STAT3dODN dose-dependently reduced pSTAT3 levels in AML cells with no significant effect of the control CpG-scrODN at the same dose.
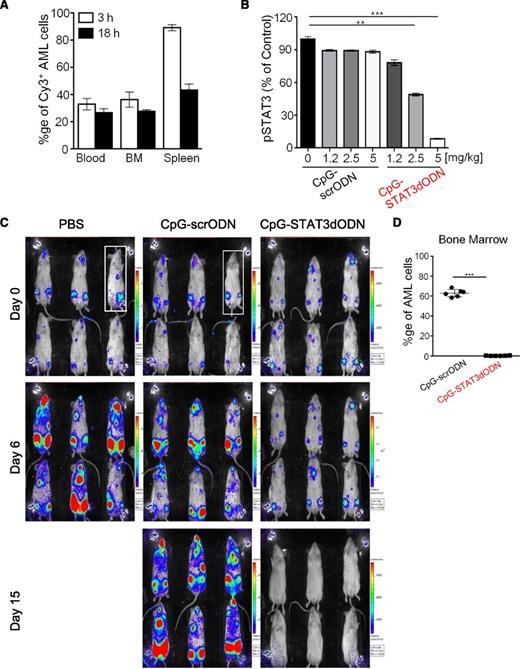
Systemic administration of CpG-STAT3dODN targets disseminated AML inducing leukemia regression. (A) Biodistribution of systemically injected CpG(D19)-STAT3dODN. MV4-11 AML cells were engrafted into immunodeficient NSG mice. Mice were then injected IV using Cy3-labeled CpG-STAT3dODN (5 mg/kg) and euthanized 3 to 18 hours later. Percentages of Cy3+/hCD45+ double-positive MV4-11 cells were assessed using flow cytometry in single-cell suspensions prepared from blood, bone marrow (BM), or spleen. Shown are representative results of 2 independent experiments using a total of 6 mice analyzed individually; means ± SEM. (B) Dose-dependent inhibition of STAT3 activity in AML in vivo. AML-bearing mice were treated 3 times using CpG-STAT3 dODN or control CpG-scrODN at indicated dosing every other day and euthanized 1 day after the last treatment. STAT3 activity at the level of Y705 phosphorylation was measured using flow cytometry; means ± SEM (n = 6). (C) Systemic delivery of CpG-STAT3dODN induces regression of human AML in mice. MV4-11ch/luc-bearing NSG mice were treated using daily IV injections (5 mg/kg) for 15 days. Tumor burden was monitored using the bioluminescent imaging (BLI) on an IVIS 100 imaging system. (D) The percentages of human CD45+ MV4-11ch/luc cells in various organs were assessed using flow cytometry at the end of the experiment (day 15); means ± SEM (n = 6). % ge, percentage.
Systemic administration of CpG-STAT3dODN targets disseminated AML inducing leukemia regression. (A) Biodistribution of systemically injected CpG(D19)-STAT3dODN. MV4-11 AML cells were engrafted into immunodeficient NSG mice. Mice were then injected IV using Cy3-labeled CpG-STAT3dODN (5 mg/kg) and euthanized 3 to 18 hours later. Percentages of Cy3+/hCD45+ double-positive MV4-11 cells were assessed using flow cytometry in single-cell suspensions prepared from blood, bone marrow (BM), or spleen. Shown are representative results of 2 independent experiments using a total of 6 mice analyzed individually; means ± SEM. (B) Dose-dependent inhibition of STAT3 activity in AML in vivo. AML-bearing mice were treated 3 times using CpG-STAT3 dODN or control CpG-scrODN at indicated dosing every other day and euthanized 1 day after the last treatment. STAT3 activity at the level of Y705 phosphorylation was measured using flow cytometry; means ± SEM (n = 6). (C) Systemic delivery of CpG-STAT3dODN induces regression of human AML in mice. MV4-11ch/luc-bearing NSG mice were treated using daily IV injections (5 mg/kg) for 15 days. Tumor burden was monitored using the bioluminescent imaging (BLI) on an IVIS 100 imaging system. (D) The percentages of human CD45+ MV4-11ch/luc cells in various organs were assessed using flow cytometry at the end of the experiment (day 15); means ± SEM (n = 6). % ge, percentage.
Based on the above observations, we decided to use daily IV injections of 5 mg/kg CpG-STAT3dODN to test its antitumor effects in mice xenotransplanted with STAT3-dependent human MV4-11ch/luc AML cells. Two weeks postengraftment, mice were injected IV daily with CpG-STAT3dODN, CpG-scrODN, or vehicle (PBS). The antitumor effect of CpG-STAT3dODN treatment started to be detectable by bioluminescent imaging at day 3 and more pronouncedly at day 6 (Figure 3C and not shown) compared with AML progression in control groups. The CpG-STAT3dODN led to regression of MV4-11ch/luc leukemia starting from day 11. Although CpG-scrODN delayed AML progression compared with PBS-treated controls, leukemia eventually progressed in all mice. In contrast, CpG-STAT3dODN reduced the percentage of bone marrow–resident hCD45+ MV4-11ch/luc cells to <1% at day 15 (Figure 3D). Together, these results suggest that CpG-STAT3dODN delivered IV reaches sufficient intracellular concentration for induction of cytotoxicity in STAT3-dependent human AML cells.
CpG-STAT3dODN reduces immunosuppressive effects of primary human AML cells
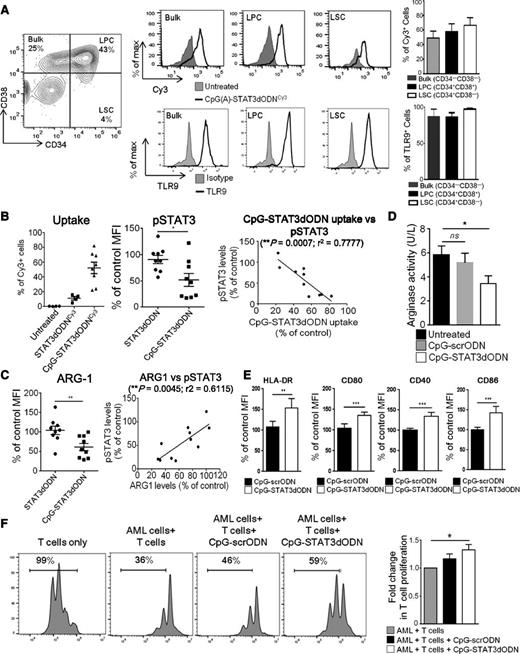
After confirming the efficient STAT3 targeting in established AML cells, we expanded our studies to primary patients’ derived leukemic cells. TLR9 expression and STAT3 activation are common in AML cells regardless of their morphologic, cytogenetic, or molecular subsets.8,37 Thus, we tested TLR9+/STAT3P+ AML blasts procured from a cytogenetically diverse group of 14 patients (Table 1). After 4-hour incubation, the majority of AML samples rapidly internalized CpG-STAT3dODN conjugates (average uptake ∼50%) including the CD34+/CD38+ leukemic progenitor cell (LPC) and CD34+/CD38− leukemic stem cell (LSC) subsets (Figure 4A top). The intracellular uptake of the conjugate correlated with high TLR9 levels in all 3 AML compartments (Figure 4A bottom). The uptake of STAT3dODN alone was negligible (<20%) as measured cytofluorimetrically (Figure 4B left). The percentage of CpG-STAT3dODN uptake correlated significantly with pSTAT3 inhibition as assessed by intracellular staining/flow cytometry (Figure 4B middle and right). Recent studies suggested that AML cells express arginase which sustains a potently immunosuppressive microenvironment.38 Arginase-1 is also 1 of the STAT3 target genes in nonmalignant, myeloid-derived suppressor cells (MDSCs).24,39 Following CpG-STAT3dODN treatment, we observed that intracellular ARG1 levels were significantly reduced in AML blasts and correlated with the extent of STAT3 inhibition (Figure 4C). Correspondingly, we showed reduced arginase activity in the supernatant of primary AML cells cultured with CpG-STAT3dODN but not scrambled controls (Figure 4D).
Clinical characteristics of AML patients’ samples
| Patient ID . | Age, y . | Sex . | Subtype . | Blasts in PB, % . | Status . | Cytogenetics . |
|---|---|---|---|---|---|---|
| 1 | 78 | M | AML-M1 | 78 | De novo | FLT3ITD+ |
| 2 | 58 | F | AML-M4 | 89 | Relapsed | FLT3ITD+ |
| 3 | 40 | F | AML-M5 | 87 | De novo | FLT3ITD+/NPM+ |
| 4 | 52 | M | AML-M1 | 87 | Refractory | Complex, multiple del./transloc., Bcr/Abl1+ |
| 5 | 43 | F | AML-NOS | 91 | De novo | FLT3ITD+ |
| 6 | 74 | F | AML-MLD (with prior MDS) | 21 | De novo | FLT3ITD+ |
| 7 | 51 | M | AML-M4 | 71 | Relapsed | Complex, multiple del./transloc. |
| 8 | 65 | M | AML-MLD (with prior MDS) | 89 | Relapsed | Normal |
| 9 | 61 | M | AML-M4 | NR | De novo | Complex, multiple del./transloc. |
| 10 | 58 | F | AML-M4 | 60 | De novo | Normal |
| 11 | 74 | M | AML-M5 | 51 | Unknown | Complex, multiple del./transloc. |
| 12 | 59 | F | AML-M1 | 56 | De novo | Normal |
| 13 | 55 | F | AML-NOS | 93 | Refractory | FLT3ITD+/NPM+ |
| 14 | 43 | F | AML-MDS | 50 | Refractory | Complex, multiple del./transloc. |
| Patient ID . | Age, y . | Sex . | Subtype . | Blasts in PB, % . | Status . | Cytogenetics . |
|---|---|---|---|---|---|---|
| 1 | 78 | M | AML-M1 | 78 | De novo | FLT3ITD+ |
| 2 | 58 | F | AML-M4 | 89 | Relapsed | FLT3ITD+ |
| 3 | 40 | F | AML-M5 | 87 | De novo | FLT3ITD+/NPM+ |
| 4 | 52 | M | AML-M1 | 87 | Refractory | Complex, multiple del./transloc., Bcr/Abl1+ |
| 5 | 43 | F | AML-NOS | 91 | De novo | FLT3ITD+ |
| 6 | 74 | F | AML-MLD (with prior MDS) | 21 | De novo | FLT3ITD+ |
| 7 | 51 | M | AML-M4 | 71 | Relapsed | Complex, multiple del./transloc. |
| 8 | 65 | M | AML-MLD (with prior MDS) | 89 | Relapsed | Normal |
| 9 | 61 | M | AML-M4 | NR | De novo | Complex, multiple del./transloc. |
| 10 | 58 | F | AML-M4 | 60 | De novo | Normal |
| 11 | 74 | M | AML-M5 | 51 | Unknown | Complex, multiple del./transloc. |
| 12 | 59 | F | AML-M1 | 56 | De novo | Normal |
| 13 | 55 | F | AML-NOS | 93 | Refractory | FLT3ITD+/NPM+ |
| 14 | 43 | F | AML-MDS | 50 | Refractory | Complex, multiple del./transloc. |
del., deletion; F, female; ID, identification; ITD, internal tandem duplication; M, male; MDS, myelodysplastic syndrome; MLD, multilineage dysplasia; NOS, not otherwise specified; NPM, nucleophosmin; PB, peripheral blood; transloc., translocation.
CpG-STAT3dODN augments immunogenicity while reducing immunosuppressive effects of patients’ AML cells. (A) CpG(D19)-STAT3dODNCy3 internalization by major compartments of AML. Patients’ leukemic blasts were incubated with CpG(D19)-STAT3dODNCy3 at 500 nM for 2 hours and analyzed by flow cytometry. Representative results from 1 of 14 analyzed samples from individual patients demonstrating gating for CD34+CD38− LSC, CD34+CD38+ LPC, and bulk leukemic cells (left) The uptake of the CpG(D19)-STAT3dODNCy3 by each AML compartment (top) and the corresponding intracellular TLR9 levels (bottom) with bar graphs summarizing results from 4 individual patients. (B) Correlation between the level of conjugate internalization and STAT3 inhibition. Shown are flow cytometric results of the internalization study (as a percentage of Cy3+ cells), assessment of pSTAT3 levels following CpG(D19)-STAT3dODN vs CpG(D19)-scrODN control treatment (compared with untreated control cells set as 100%) and pairwise analysis of correlation between levels of uptake and pSTAT3 reduction (n = 10). (C) CpG(D19)-STAT3dODN reduced Arginase-1 expression in primary AML cells. (Left) Intracellular levels of ARG1 were assessed using flow cytometry after treatment using indicated conjugates compared with untreated control set as 100%. (Right) Pairwise analysis of correlation between levels of ARG1 and pSTAT3 reduction in individual patients’ samples (n = 11). (D) Decrease in arginase activity in supernatants from cultured patients’ AML cells treated as indicated for 72 hours. Results from the analysis of 6 individual patients’ samples. (E) The surface expression of HLA-DR or costimulatory CD86, CD80, CD40 molecules was determined using flow cytometry. Shown are means ± SEM using samples from 5 individual patients. (F) Partial reduction of the immunosuppressive effect of primary AML blasts on T-cell proliferation. Primary AML cells pretreated in vitro using CpG-STAT3dODN were later cocultured with allogeneic T cells at 1:1 for 3 days. T-cell proliferation was assessed using CFSE dilution assay; percentages of dividing T cells past the parental generation are indicated. Shown are representative results from 1 of 5 independent experiments; means ± SEM (n = 5).
CpG-STAT3dODN augments immunogenicity while reducing immunosuppressive effects of patients’ AML cells. (A) CpG(D19)-STAT3dODNCy3 internalization by major compartments of AML. Patients’ leukemic blasts were incubated with CpG(D19)-STAT3dODNCy3 at 500 nM for 2 hours and analyzed by flow cytometry. Representative results from 1 of 14 analyzed samples from individual patients demonstrating gating for CD34+CD38− LSC, CD34+CD38+ LPC, and bulk leukemic cells (left) The uptake of the CpG(D19)-STAT3dODNCy3 by each AML compartment (top) and the corresponding intracellular TLR9 levels (bottom) with bar graphs summarizing results from 4 individual patients. (B) Correlation between the level of conjugate internalization and STAT3 inhibition. Shown are flow cytometric results of the internalization study (as a percentage of Cy3+ cells), assessment of pSTAT3 levels following CpG(D19)-STAT3dODN vs CpG(D19)-scrODN control treatment (compared with untreated control cells set as 100%) and pairwise analysis of correlation between levels of uptake and pSTAT3 reduction (n = 10). (C) CpG(D19)-STAT3dODN reduced Arginase-1 expression in primary AML cells. (Left) Intracellular levels of ARG1 were assessed using flow cytometry after treatment using indicated conjugates compared with untreated control set as 100%. (Right) Pairwise analysis of correlation between levels of ARG1 and pSTAT3 reduction in individual patients’ samples (n = 11). (D) Decrease in arginase activity in supernatants from cultured patients’ AML cells treated as indicated for 72 hours. Results from the analysis of 6 individual patients’ samples. (E) The surface expression of HLA-DR or costimulatory CD86, CD80, CD40 molecules was determined using flow cytometry. Shown are means ± SEM using samples from 5 individual patients. (F) Partial reduction of the immunosuppressive effect of primary AML blasts on T-cell proliferation. Primary AML cells pretreated in vitro using CpG-STAT3dODN were later cocultured with allogeneic T cells at 1:1 for 3 days. T-cell proliferation was assessed using CFSE dilution assay; percentages of dividing T cells past the parental generation are indicated. Shown are representative results from 1 of 5 independent experiments; means ± SEM (n = 5).
We have recently shown that inhibiting STAT3 in leukemia cells in mice can induce immunogenic effects through increased presentation of tumor-associated antigens.12 In cultured primary human AML cells, CpG-STAT3dODN led to significant but only moderate upregulation of the surface expression of HLA-DR and costimulatory molecules, such as CD86, CD80, and CD40 (Figure 4E). This is likely a result of the reduced STAT3 activity compared with AML cells in vivo.12 We next evaluated whether CpG-STAT3dODN will improve proliferation of allogeneic T cells cocultured with primary AML blasts. As shown in Figure 4F, pretreatment of patients’ derived AML cells with CpG-STAT3dODN but not CpG-scrODN moderately enhanced CD3-driven T-cell proliferation. These results imply that the CpG-STAT3dODN strategy can have a 2-pronged effect against AML with direct cytotoxicity, at least against a subset of STAT3-dependent AML, and immunostimulatory effects are likely to occur in diverse types of leukemia.
CpG-STAT3dODN induces regression of syngeneic AML in mice
To assess therapeutic potential of CpG-STAT3dODN in immunocompetent mice, we used the syngeneic model of Cbfb/MYH11/Mpl+ leukemia (CMM). Mice with circulating GFP+/c-kit+ CMM leukemia cells (>1% AML cells) were treated 6 times every other day using IV injections of 5 mg/kg CpG-STAT3dODN, STAT3dODN alone, control CpG-scrODN, or PBS. As shown in Figure 5A, CpG-STAT3dODN decreased AML burden in spleen or bone marrow from the average 50% to ∼5% or <10%, respectively. The unconjugated STAT3dODN was significantly less effective, reducing tumor burden by ∼45% to 50%, whereas the antitumor effect of CpG-scrODN was negligible (Figure 5A). These results underscore the synergistic effect of combining CpG ODN with the STAT3 decoy inhibitor into a single bifunctional molecule. The conjugates used in these experiments used the CpG-1668 ODN for optimal stimulation of mouse immune cells, however, similar results were observed using CpG-D19–based conjugates (supplemental Figure 7). We also verified these results by examining spleens harvested from CMM-bearing mice posttreatment. Although CpG-scrODN failed to reduce splenomegaly, the size of spleens in mice treated using CpG-STAT3dODN decreased fourfold (Figure 5B). To assess whether these effects correspond to the level of STAT3 inhibition, we measured levels of activated STAT3 (pSTAT3), total STAT3, and Bcl-XL. Both STAT3 and Bcl-XL are downstream targets of STAT3 signaling.18,40 Western blot analysis confirmed that CpG-STAT3dODN, but not CpG-scrODN, significantly downregulated pSTAT3 levels as well as total STAT3 and Bcl-XL levels in both bone marrow and spleen samples (Figure 5C-D; supplemental Figure 8).
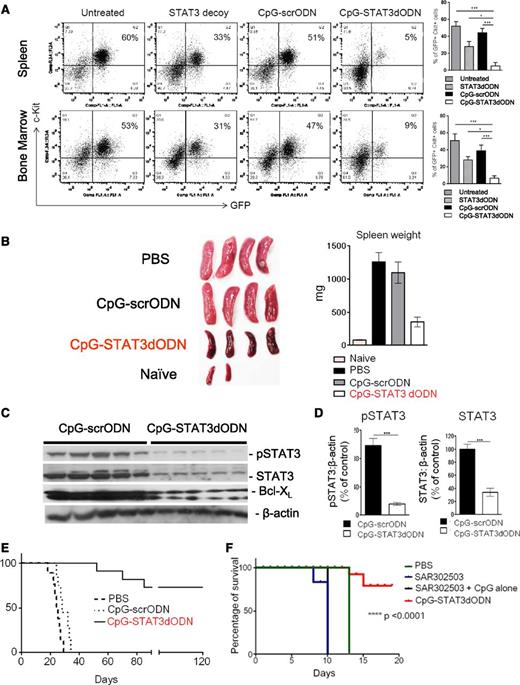
Systemic CpG-STAT3dODN treatment induces regression of syngeneic Cbfb/Myh11/Mpl leukemia in mice. C57BL/6 mice were injected IV with 250 000 fresh CMM cells. After 7 to 10 days when tumors were engrafted (ranging from 1% to 5% of AML cells in blood), mice were injected 6 times with CpG(1668)-STAT3dODN or control CpG(1668)-scrODN (5 mg/kg) every other day and euthanized a day after the last treatment. (A) Reduction of leukemia burden in spleen (top) or bone marrow (bottom) after treatment using CpG-STAT3dODN, STAT3dODN alone, or control CpG-scrODN. (Left) The representative flow cytometric results of GFP+c-Kit+ AML cells from various groups of mice. Shown are combined results from 6 mice per group; means ± SEM. Statistically significant differences between groups are indicated with asterisks. (B) Spleen sizes in various group of mice treated as described in panel A. (Left) Representative image of spleens harvested from mice at the end of experiment. (Right) Combined results showing spleen weight for each treatment group. (C) STAT3 inhibition by CpG-STAT3dOND results in decreased pSTAT3 and total STAT3 levels as well as in reduction of BCL-XL. The western blot analysis of bone marrow from CpG-STAT3dODN– or control CpG-scrODN–treated mice. Similar results were derived from 3 independent experiments. (D) Band intensities were quantified densitometrically using ImageJ version 1.46 software based on identically exposed images. Statistically significant differences between CpG-STAT3 dODN and CpG-scrODN–treated groups are indicated by asterisks (**P < .003; ***P < .0001). (E) Systemic administration of CpG-STAT3dODN reduces leukemia-initiating potential in the CMM model. CMM cells were isolated from bone marrow of primary recipient mice treated as described in panel A. Magnetically enriched c-Kit+ AML cells pooled from CpG-STAT3dODN, CpG-scrODN, or untreated mice were combined and counted, and identical cell numbers were injected into secondary recipient mice. Long-term survival of the secondary transplant recipients is shown; n = 10 for each group. (F) Systemic STAT3 inhibition using a small-molecule Jak2/STAT3 inhibitor (SAR302503/TG101348) fails to improve survival of AML-bearing mice. Mice engrafted with CMM leukemia as described in panel A were treated 6 times every other day using CpG-STAT3dODN (5 mg/kg by IV injections), SAR302503 alone (100 mg/kg by oral gavage), or SAR302503 combined with CpG ODN (5 mg/kg by IV injections) vs control mice (treated with PBS); n = 6 in each treatment group.
Systemic CpG-STAT3dODN treatment induces regression of syngeneic Cbfb/Myh11/Mpl leukemia in mice. C57BL/6 mice were injected IV with 250 000 fresh CMM cells. After 7 to 10 days when tumors were engrafted (ranging from 1% to 5% of AML cells in blood), mice were injected 6 times with CpG(1668)-STAT3dODN or control CpG(1668)-scrODN (5 mg/kg) every other day and euthanized a day after the last treatment. (A) Reduction of leukemia burden in spleen (top) or bone marrow (bottom) after treatment using CpG-STAT3dODN, STAT3dODN alone, or control CpG-scrODN. (Left) The representative flow cytometric results of GFP+c-Kit+ AML cells from various groups of mice. Shown are combined results from 6 mice per group; means ± SEM. Statistically significant differences between groups are indicated with asterisks. (B) Spleen sizes in various group of mice treated as described in panel A. (Left) Representative image of spleens harvested from mice at the end of experiment. (Right) Combined results showing spleen weight for each treatment group. (C) STAT3 inhibition by CpG-STAT3dOND results in decreased pSTAT3 and total STAT3 levels as well as in reduction of BCL-XL. The western blot analysis of bone marrow from CpG-STAT3dODN– or control CpG-scrODN–treated mice. Similar results were derived from 3 independent experiments. (D) Band intensities were quantified densitometrically using ImageJ version 1.46 software based on identically exposed images. Statistically significant differences between CpG-STAT3 dODN and CpG-scrODN–treated groups are indicated by asterisks (**P < .003; ***P < .0001). (E) Systemic administration of CpG-STAT3dODN reduces leukemia-initiating potential in the CMM model. CMM cells were isolated from bone marrow of primary recipient mice treated as described in panel A. Magnetically enriched c-Kit+ AML cells pooled from CpG-STAT3dODN, CpG-scrODN, or untreated mice were combined and counted, and identical cell numbers were injected into secondary recipient mice. Long-term survival of the secondary transplant recipients is shown; n = 10 for each group. (F) Systemic STAT3 inhibition using a small-molecule Jak2/STAT3 inhibitor (SAR302503/TG101348) fails to improve survival of AML-bearing mice. Mice engrafted with CMM leukemia as described in panel A were treated 6 times every other day using CpG-STAT3dODN (5 mg/kg by IV injections), SAR302503 alone (100 mg/kg by oral gavage), or SAR302503 combined with CpG ODN (5 mg/kg by IV injections) vs control mice (treated with PBS); n = 6 in each treatment group.
Finally, we assessed whether leukemia regression induced by CpG-STAT3dODN treatment could eliminate LSCs which drive AML chemoresistance and relapse.41 For this purpose, we isolated bone marrow–resident CMM cells after 6 IV injections of CpG-STAT3dODN, CpG-scrODN, or only PBS. The same numbers of viable AML cells were later transplanted into naive, secondary recipient mice. The majority (7 of 10) of mice that were engrafted with CpG-STAT3dODN–treated AML cells survived for >120 days with no signs of leukemia whereas control CpG-scrODN– and vehicle-treated recipients developed lethal AML in 35 days (Figure 5E). Taken together, our findings suggests that systemic administration of CpG-STAT3dODN efficiently targets the LSC compartment in disseminated CMM leukemia, thereby allowing for complete tumor regression and long-term animal survival. Importantly, CpG-STAT3dODN did not induce toxicity in normal immune cells and hematopoietic stem cells, even after extended systemic administration for 2 months (supplemental Figure 6). Mice tolerated well a maximum dose of 87.5 mg/kg per week (supplemental Figure 9), and long-term treatment did not result in autoimmunity related to STAT3 ablation, such as ileitis (supplemental Figure 10).42
Next, we compared the therapeutic efficacy of CpG-STAT3dODN to clinically relevant small-molecule inhibitor of Jak/STAT3 signaling, SAR302508/TG101348, which potently inhibits STAT3 activity in preclinical tumor models and in human subjects.43,44 As expected, SAR302503 had a potent cytotoxic effect on mouse CMM cells in vitro (supplemental Figure 11). However, in contrast to CpG-STAT3dODN, it failed to induce antitumor effects against the same AML model in vivo when used alone or in combination with CpG ODN (Figure 5F). We assume that this discrepancy may reflect impaired immune activation due to the inhibition of Janus kinase 2 (JAK2)/STAT3-mediated signaling in T cells.45
Increased immunogenicity of leukemic cells is critical for the antitumor efficacy of CpG-STAT3dODN
CpG-STAT3dODN showed immunogenic effects on a variety of patients’ primary AML cells. To assess the contribution of direct cytotoxicity induced by CpG-STAT3dODN to leukemia regression, we transplanted CMM cells into immunodeficient NSG mice and then injected them IV using 6 injections of CpG-STAT3dODN, CpG-scrODN, or vehicle every other day. As shown in Figure 6A, lack of the adaptive immunity reduced but did not completely eliminate effects of CpG-STAT3dODN which decreased tumor burden by 20% to 30%. Compared with an effect of CpG-STAT3dODN on xenotransplanted MV4-11 AML (Figure 3C), cytotoxic effects of the conjugate on CMM cells were limited likely due to less frequent dosing or lesser dependence of CMM cells on STAT3 than STAT5 for their survival.12
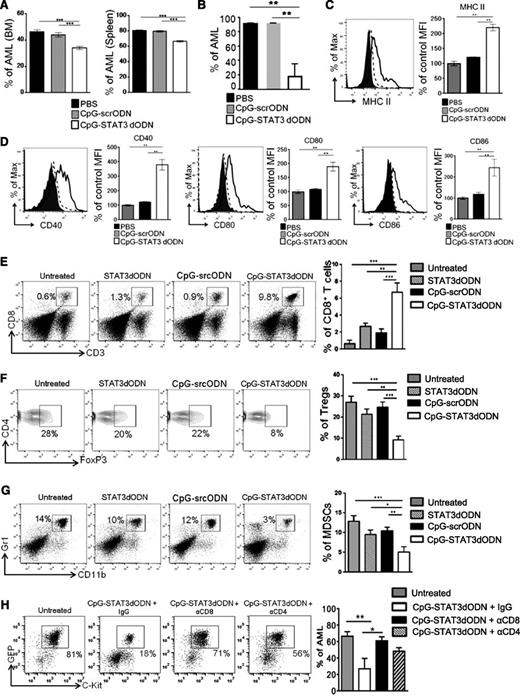
Antileukemic effect of CpG-STAT3dODN in vivo is immune-mediated and depends on the induction of AML immunogenicity. (A-B) NOD/SCID/IL-2RγKO (A) or Tlr9−/− mice (B) were challenged IV using CMM cells and treated using CpG(1668)-STAT3dODN, CpG(1668)-scrODN, or PBS as in Figure 5A. The percentages of GFP+c-Kit+ AML cells in different organs were determined by flow cytometry. Shown are means ± SEM from 2 independent experiments; n = 6 per group for each experiment. Statistically significant differences were indicated by asterisks. (C-D) CMM-bearing C57BL/6 mice were treated using CpG-STAT3dODN, CpG-scrODN, or PBS siRNA as in panel A. The surface expression of MHC class II (C) and costimulatory molecules CD40, CD80, CD86 (D) on splenic AML cells was assessed by flow cytometry. Shown are representative histogram overlays and bar graphs summarizing results from each group of mice (n = 6); mean ± SEM. (E-G) Systemic administration of CpG(B)-STAT3dODN treatment induces infiltration of CD8+ T cells while reducing percentages of Tregs and MDSCs in spleens of AML-bearing mice. Mice were treated as described in Figure 5A. Percentages of immune cells were assessed using flow cytometry in cell suspensions prepared from spleens of treated mice. Shown are representative histograms and combined results in bar graphs; means ± SEM (n = 6). Statistically significant differences were indicated by asterisks. (H) CD8+ and CD4+ T-cell populations are required for the antitumor effect of CpG-STAT3dODN. C57BL/6 mice were injected intraperitoneally with neutralizing antibodies specific to CD8 (2.43), CD4 (GK1.5), control immunoglobulin G (IgG) or left untreated and then challenged IV with CMM leukemia as in Figure 5A. Mice with established leukemia were injected 6 times every other day using CpG-STAT3dODN or left untreated. Percentages of AML cells in spleens were determined by flow cytometry; shown are representative dot plots and the combined results from 1 experiment using 6 mice per each experimental group; means ± SEM.
Antileukemic effect of CpG-STAT3dODN in vivo is immune-mediated and depends on the induction of AML immunogenicity. (A-B) NOD/SCID/IL-2RγKO (A) or Tlr9−/− mice (B) were challenged IV using CMM cells and treated using CpG(1668)-STAT3dODN, CpG(1668)-scrODN, or PBS as in Figure 5A. The percentages of GFP+c-Kit+ AML cells in different organs were determined by flow cytometry. Shown are means ± SEM from 2 independent experiments; n = 6 per group for each experiment. Statistically significant differences were indicated by asterisks. (C-D) CMM-bearing C57BL/6 mice were treated using CpG-STAT3dODN, CpG-scrODN, or PBS siRNA as in panel A. The surface expression of MHC class II (C) and costimulatory molecules CD40, CD80, CD86 (D) on splenic AML cells was assessed by flow cytometry. Shown are representative histogram overlays and bar graphs summarizing results from each group of mice (n = 6); mean ± SEM. (E-G) Systemic administration of CpG(B)-STAT3dODN treatment induces infiltration of CD8+ T cells while reducing percentages of Tregs and MDSCs in spleens of AML-bearing mice. Mice were treated as described in Figure 5A. Percentages of immune cells were assessed using flow cytometry in cell suspensions prepared from spleens of treated mice. Shown are representative histograms and combined results in bar graphs; means ± SEM (n = 6). Statistically significant differences were indicated by asterisks. (H) CD8+ and CD4+ T-cell populations are required for the antitumor effect of CpG-STAT3dODN. C57BL/6 mice were injected intraperitoneally with neutralizing antibodies specific to CD8 (2.43), CD4 (GK1.5), control immunoglobulin G (IgG) or left untreated and then challenged IV with CMM leukemia as in Figure 5A. Mice with established leukemia were injected 6 times every other day using CpG-STAT3dODN or left untreated. Percentages of AML cells in spleens were determined by flow cytometry; shown are representative dot plots and the combined results from 1 experiment using 6 mice per each experimental group; means ± SEM.
CpG-STAT3dODN showed comparable antileukemic efficacy in Tlr9-deficient as in wild-type mice whereas CpG-scrODN control treatment had no effect (Figure 6B). Given that antigen-presenting cells in Tlr9-deficient mice lack sensitivity to CpG, these results indicate that CpG-STAT3dODN triggers AML immunogenicity and antigen presentation independently from specialized host’s antigen-presenting cells.12,21 In fact, similar to our in vitro experiments on human AML blasts, CpG-STAT3dODN, but not CpG-scrODN, induced upregulation of MHC class II (Figure 6C), CD40, CD80, and CD86 molecules (Figure 6D) on splenic GFP+ AML cells and on normal DCs (GFP−) isolated from the same mice (supplemental Figure 12). The increased AML immunogenicity correlated with dramatic changes in the composition of the leukemia microenvironment. We observed a >10-fold increase in CD8+ T-cell infiltration into leukemia reservoirs, such as spleen, after CpG-STAT3dODN treatment compared with untreated control (Figure 6E). Increased numbers of effector T cells coincided with reduction in numbers of immunosuppressive regulatory T cells (Tregs; CD4+FoxP3+) (Figure 6F) and MDSCs (CD11b+Gr1+) (Figure 6G). Although STAT3dODN alone and, to a lesser extent CpG-scrODN, weakly induced CD8+ T-cell infiltration, both treatments failed to reduce Treg and MDSC percentages. Finally, the antibody-mediated T-cell depletion of CD8 or CD4 T cells confirmed the role of both populations in the antitumor effect of CpG-STAT3dODN (Figure 6H). Overall, these results underscore the advantage of dual-function CpG-STAT3dODN over immunostimulatory CpG ODNs or nonimmunogenic STAT3 inhibitors.
Discussion
We present here a clinically relevant strategy for systemic and cell-selective delivery of a STAT3 decoy inhibitor to nonmalignant and leukemic myeloid cells in vivo. The unformulated CpG-STAT3dODN conjugates injected IV reduce STAT3 activity in leukemic cells in mice, thereby resulting in regression of STAT3-dependent human AML and complete eradication of leukemia in immunocompetent mice. Therapeutic efficacy of CpG-STAT3dODN against human and mouse AML in vivo exceeds these of STAT3 decoy alone, our earlier siRNA-based strategy12 or even small-molecule JAK/STAT3 inhibitor. The IV dosing of CpG-STAT3dODN in both human and mouse AML models compares favorably with the dose range of nonimmunogenic and nonselective STAT3 inhibitors, such as antisense oligonucleotides.46 The CpG-assisted delivery of STAT3dODN expands the therapeutic potential of decoy strategy, which has been limited to certain types of solid tumors, mainly head and neck cancers.28,47,48 CpG-STAT3dODN overcomes obstacles in oligonucleotide delivery to hematopoietic cells, which are challenging targets for lipid- and polymer-based approaches.49 We do not exclude that the antitumor effect of CpG-STAT3dODN is partly derived from concomitant blocking of STAT3 together with STAT1 which reportedly have overlapping tumor-promoting roles in leukemia and certain other tumors.31-33 At the same time, CpG-STAT3dODN does not impede but promotes CD8+ T-cell recruitment into leukemia-bearing organs likely driven by STAT1 signaling in effector lymphocytes.50,51 In addition, myeloid cell-selective STAT3 inhibition prevents interference with STAT3-dependent generation of memory T cells and long-term antitumor immunity.52,53
The majority of AML cells are arrested at the early stage of myeloid cell differentiation, creating an opportunity to initiate and redirect their maturation toward DCs. Significant effort was dedicated to AML immunotherapies based on ex vivo–generated leukemic DCs with the ability to present leukemic antigens to T cells.54-56 However, vaccination strategies using leukemic DCs failed to achieve clinical efficacy when confronted with the immunosuppressive AML microenvironment.38,57-59 Results from our in vitro experiments on patients’ AML blasts indicate the feasibility of using CpG-STAT3dODN to trigger immunogenicity and reduce arginase-dependent immunosuppression in primary human AML representing different subtypes and cytogenetic characteristics. STAT3 blocking/TLR9 triggering augmented expression of MHC class II and costimulatory molecules as previously described in nonmalignant myeloid cells, such as DCs.60-63 The direct cytotoxic effects of STAT3 inhibition, although detectable, were limited by alternative survival signaling in AML cells.12,20 The simplicity of the CpG-dODN design allows for further adaptation of this strategy to targeting other tumorigenic and/or immunosuppressive transcription factors beyond STAT3, such as STAT5 and NF-κB, currently tested by our group.
Our CpG-STAT3dODN strategy allows for the temporarily lifting of the STAT3-mediated immune checkpoint control and the triggering of unrestricted TLR9-induced and CD8/CD4 T-cell–mediated immune responses.29 The reversibility of STAT3 decoy-mediated inhibition in normal myeloid cells limits the risk of autoimmune manifestations or desensitization/tolerization of immune cells after repeated TLR stimulation.64 The combination of directly cytotoxic and immune-mediated effects against AML cells can generate specific and long-term antitumor effects, less likely to be limited by the development of therapeutic resistance. CpG-STAT3dODN did not induce toxic effects in human and/or mouse immune cells in vitro and in vivo, and our recent dose assessment showed that mice tolerate well IV injections of CpG-STAT3dODN. Therefore, we believe that our findings provide evidence supporting further development of the dual-function CpG-STAT3dODN for treatment of AML and potentially other TLR9+ malignancies, such as B-cell lymphoma or certain solid cancers.18,65
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Drs J. Barrett, P. Muranski, and S. Ito (National Institutes of Health) for valuable suggestions, and to the staff at the Analytical Cytometry, Pathology and Animal Resource Cores (City of Hope) for their support.
This work was supported in part by the National Cancer Institute/National Institutes of Health award number R01CA155367 (M.K.), R01CA178387 (Y.-H.K.), P30CA033572 (COH), the STOP-CANCER Allison-Tovo-Dwyer Memorial Career-Development Award (M.K.), the V-Foundation for Cancer Research Scholar (Y.-H.K.), and the American Cancer Society, Research Scholar Grant 123278-RSG-12-140-01-CSM (Y.-H.K).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: Q.Z., D.M.S.H., D.M., S.N., and M.K. designed the study; Q.Z., D.M.S.H., P.D., D.M., S.N., Q.C., H.W., and X.Z. conducted experiments; Q.Z., D.M.S.H., M.M., S.F., Y.-H.K., G.M., and M.K. analyzed data; P.S., R.B., B.Z., Y.-H.K., and G.M. provided reagents; and Q.Z. and M.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcin Kortylewski, City of Hope, Beckman Center, Room 3111, 1500 East Duarte Rd, Duarte, CA 91010; e-mail: mkortylewski@coh.org.
References
Author notes
Q.Z., D.M.S.H., P.D., and D.M. contributed equally.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal