Key Points
Genetic and nongenetic determiners of MPV substantially differ between males and females in a large population-based study.
MPV in males is significantly determined by the traditional CVRFs, and males with higher MPV are at higher risk of death.
Abstract
Mean platelet volume (MPV), a measure of platelet size, is a potential biological marker of platelet function. To date, a comprehensive analysis including known genetic and nongenetic factors that determine MPV is still lacking. MPV has been evaluated in 15 010 individuals from the population-based Gutenberg Health Study. Genetic information was available for 4175 individuals. Our results showed that age (β, 0.0346; 95% confidence interval [CI], 0.0255 to 0.0436), cardiovascular risk factors (CVRFs) such as smoking (β, 0.178; 95% CI, 0.128 to 0.229), hypertension (β, 0.05; 95% CI, 0.00289 to .0981), and high glucose level (β, 0.00179; 95% CI, 0.0006 to 0.00299) were linked with higher MPV in males only. Intake of oral contraceptives (β, 0.150; 95% CI, 0.0649 to 0.236) and menstruation (β, 0.123; 95% CI, 0.0231 to 0.224) were strongly associated with higher MPV in females. Seven single nucleotide polymorphisms (SNPs) for females and 4 SNPs for males were associated with higher MPV. The full model, including age, CVRFs, laboratory parameters, medications, and genetic variation, explained 20.4% of the MPV variance in females and 18.6% in males. The curves of cumulative mortality, stratified for sex, showed worse survival for males only with MPV >9.96 fL vs MPV ≤9.96 fL (P < .0001). This study provides evidence for heterogeneity in the profile of determinants for MPV between sexes. The observed interactions between genetic variability, CVRFs, and MPV and its association with the development of cardiovascular disease or thrombotic risk need to be further investigated.
Introduction
Platelets play a key role in both hemostasis and thrombosis and, according to recent findings, in inflammation, atherogenesis, and cancer metastasis.1 Mean platelet volume (MPV), a measure of platelet size, is a potential biological marker of platelet function and is commonly available in the clinical practice at relatively low cost. Several cytokines and growth factors may affect megakaryocytopoiesis and release of larger platelets. In addition, changes in platelet volume may also occur at the sites of activation with changes in platelet shape.2 Larger platelets are metabolically and enzymatically more active and have higher thrombotic potential and higher expression of platelet surface activation markers.3-5 Higher MPV was shown to be associated with traditional cardiovascular risk factors6 such as hypertension,7 diabetes,8 obesity,9 hypercholesterolemia,10 and smoking11 and is correlated with an increased risk for both arterial12,13 and venous thrombosis.14 Patients with preexisting coronary artery disease and increased MPV are at higher risk of myocardial infarction (MI).15 Furthermore, higher MPV implied an increased risk of vascular mortality, particularly of ischemic heart disease, in a large population study.16
It has been recognized for almost 40 years that sex may influence platelet biology.17,18 Therefore, the determinants of MPV may vary between males and females. Recent studies investigating the relation between MPV and vascular risk factors report higher MPV in females except for a Danish population based-study demonstrating higher MPV in males.12,14,16,19
Both genetic and nongenetic factors may influence the variation of MPV. White blood cell (WBC) counts, sex, and age have recently been identified as major determinants of MPV heterogeneity.19 A genome-wide association study (GWAS) identified eight chromosomal regions with 22 independent single nucleotide polymorphisms (SNPs) that influence MPV across cohorts.20 One of these SNPs, rs342293 (FLJ36031; PIC3CG) on chromosome 7q22.3 was recently associated with larger MPV and with higher risk for adverse cardiovascular outcome in patients with coronary artery disease who were undergoing percutaneous coronary intervention.21
To date, a comprehensive, combined analysis that includes known genetic and nongenetic factors that determine MPV is still lacking. In addition, sex-specific information regarding the relation of MPV and cardiovascular risk factors is limited. The Gutenberg Health Study (GHS) offers the potential to investigate sex-specific disparities in MPV determinants. Because of the observational and representative character of this study, the continuum from healthy individuals to those with cardiovascular disease such as coronary heart disease, peripheral artery disease, history of MI, and heart failure is present in the study sample. The setting offers the advantage of exploring both genetic and nongenetic influences on MPV variation in a comprehensively phenotyped population sample with a standardized setting for MPV measurement.22
Research design and methods
Population sample
MPV was determined in 15 010 participants enrolled in the GHS between April 2007 and April 2012 at their baseline examination. The cohort was part of a population-based, prospective, observational, single-center study in western mid-Germany conceptualized primarily for studying cardiovascular disease. Residents of Mainz and Mainz-Bingen County age 35 to 74 years were randomly selected from the registries of the local governmental registry offices with stratification for sex, residence (urban vs rural), and decade of age. All participants underwent a 5-hour examination at the study center and followed standard operating procedures provided by certified medical technical assistants. Details of the study design were published earlier.23 The project was approved by the local ethics committee, and the study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants before they entered the study.
Blood sampling and laboratory measurements
Venous blood sampling was performed by using tubes containing K3-EDTA. Platelet parameters (count and MPV) were automatically determined on an ADVIA 120 Hematology System (Siemens, Erlangen, Germany) between 30 and 90 minutes after blood sampling.
Internal quality control of automated blood counts was performed by using the TESTpoint controls for the ADVIA 120 Hematology System (Siemens). Results had to be within the limits indicated for the respective control material. Coefficients of variation for MPV ranged between 0.8% and 2.2% over time with a mean of 1.4%. The moving average analysis on MPV performed over the duration of the study is presented in supplemental Figure 1 (available on the Blood Web site). Results have been obtained with three ADVIA 120 analyzers (Siemens) at the Institute for Clinical Chemistry and Laboratory Medicine, University Medical Center Mainz, in Mainz, Germany. To continuously validate and align the measuring systems, 10 random blood samples were analyzed on all 3 instruments on a monthly basis, and the results were evaluated. The inter-instrument coefficient of variation of MPV varied between 1.8% and 4.1% during the study period (supplemental Figure 2). Inter-instrument coefficients of variation <5% were considered acceptable.
Biochemical analysis of blood glucose and C-reactive protein (CRP) measured in plasma and cholesterol, high-density lipoprotein, low-density lipoprotein, cholesterol, and triglycerides measured in serum were determined on the day of sampling by routine methods. All laboratory measurements were performed in the central laboratory of the University Medical Center in Mainz.
Selection, genotyping, and imputation of SNPs
MPV-related SNPs were selected from the catalog of published GWASs with the term “mean platelet volume”24 and by a systematic search of PubMed using the keywords “MPV”, “mean platelet volume”, “SNPs”, and “genetic variants”. Overall, 36 SNPs were selected (supplemental Table 1) from the available data, which were described by large GWASs for MPV.20,25,26
SNP information was taken from the genetic data available from the GHS. Genotyping was conducted on Affymetrix Genome-Wide Human SNP 6.0 arrays (Affymetrix, Santa Clara, CA) according to the manufacturer´s recommendations. After a quality control review, genetic data were available for analysis in 4175 individuals. Before genotype imputation, SNPs showing significant (P < 10−4) deviation from the Hardy-Weinberg equilibrium with minor allele frequency <1% or having a genotyping call rate <98% were excluded. A two-step process was performed for imputation. First, prephasing was performed by using MACH v1.0.18c software. Second, genotypes were imputed by using the reference panel 1000G Phase I Integrated Release Version 2 Haplotypes (2010-11 data freeze, 2012-02-14 haplotypes), and minimac (release 2012-03-14) was used. The imputation results were filtered by using a minimum imputation quality score r2 > 0.3 and a minor allele frequency threshold of 2%. Imputation results were summarized as allele dosage, defined as the expected number of copies of the minor allele for each SNP (values of 0, 1, and 2), and genotype. A ratio of observed to expected variance of the dosage statistic for each SNP was calculated for imputation quality.
Data management and statistical analysis
Safe and efficient data management was ensured by acquiring, storing, and analyzing all data electronically. All data underwent quality control with a review for completeness and plausibility by using predefined algorithms and criteria from a central data management unit.
Distribution of MPV is presented as the median with interquartile range and was tested by using the Mann-Whitney U test. Normally distributed continuous variables were described by using mean ± standard deviation and tested with the t test, whereas categorical variables were expressed as absolute numbers and percentages and tested with the χ2 test if needed. The population sample was categorized according to the 95th percentile of MPV in the reference group. The reference group was a subsample of the population sample and was defined as individuals without known clinically established disease (ie, MI, coronary artery disease, stroke, peripheral arterial disease, venous thromboembolism, atrial fibrillation, chronic heart failure, cancer, kidney disease, liver disease, chronic obstructive pulmonary disease, and anemia) or traditional cardiovascular risk factors (ie, hypertension, obesity, dyslipidemia, diabetes mellitus, smoking, and family history of MI or stroke). For the definition of traditional cardiovascular risk factors and categorization of medications, please see supplemental Data. Relations between continuous variables were explored by using Pearson’s correlation coefficients. Quantile regression was used to describe median MPV levels. Multivariable linear regression analysis was used to identify determinants of MPV. Because of the explorative character of the analysis, a significance threshold was not defined for P values. P values should be interpreted as a continuous measure of statistical evidence. The analysis of prospective mortality data with censoring is presented by using plots of cumulative mortality with log-rank test and by Cox proportional hazards models.
Results
Participant characteristics
Table 1 shows continuous biomarkers of cardiovascular risk stratified for the 95th percentile of the reference group, which was determined as 9.9 fL for males, 10.1 fL for females, and 10.1 fL in the overall sample. WBC count, CRP, fibrinogen, and glucose and triglyceride levels were significantly higher, whereas platelet count, low-density lipoprotein, high-density lipoprotein, and total cholesterol were significantly lower in the group of participants with MPV above the reference limit.
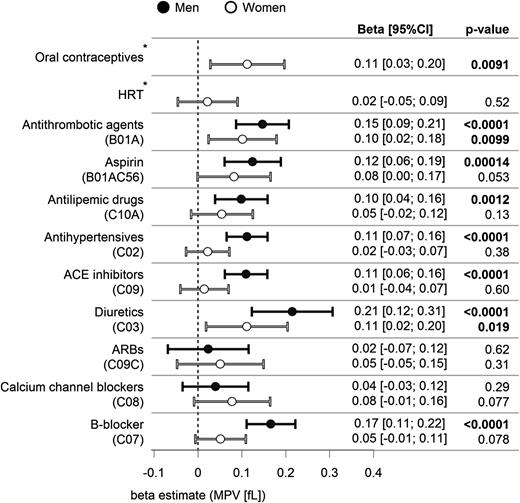
Traditional cardiovascular risk factors (CVRFs) (ie, diabetes and smoking) were associated with higher MPV both in males and females (supplemental Figure 3). In addition, obesity was associated with higher MPV in males only. Figure 1 shows the associations between medication and MPV in separate single linear regression models for each drug adjusted for age. Antithrombotic agents, including aspirin and diuretics, were associated with higher MPV in both males and females. Furthermore, antilipemic agents, antihypertensives, angiotensin-converting enzyme inhibitors, and beta blockers were associated with higher MPV in males. Interestingly, oral contraceptives, but not hormone replacement therapy, showed a significant association with higher MPV in females. Sex-specific multivariable linear regression models adjusted for age, classical CVRFs, medication, and laboratory parameters (Table 2) described 11.9% of MPV variation in both males and females. WBC count and fibrinogen were positively associated with MPV, whereas platelet count and CRP were negatively associated. In male participants, hypertension, smoking, and glucose levels were positively associated with MPV (Table 2), whereas in females, a positive association with MPV was observed for menstruation (β, 0.123; 95% CI, 0.0231 to 0.224; P = .016) and oral contraceptives (β, 0.150; 95% CI, 0.0065 to 0.236; P = .00056).
Medical treatment and MPV. Estimates of β with 95% CIs for each drug from linear regression modeling for MPV adjusted for age. Results are displayed with stratification for males (black) and females (white). *Self-reported information. ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; HRT, hormone replacement therapy.
Medical treatment and MPV. Estimates of β with 95% CIs for each drug from linear regression modeling for MPV adjusted for age. Results are displayed with stratification for males (black) and females (white). *Self-reported information. ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; HRT, hormone replacement therapy.
Age- and sex-specific distribution of MPV
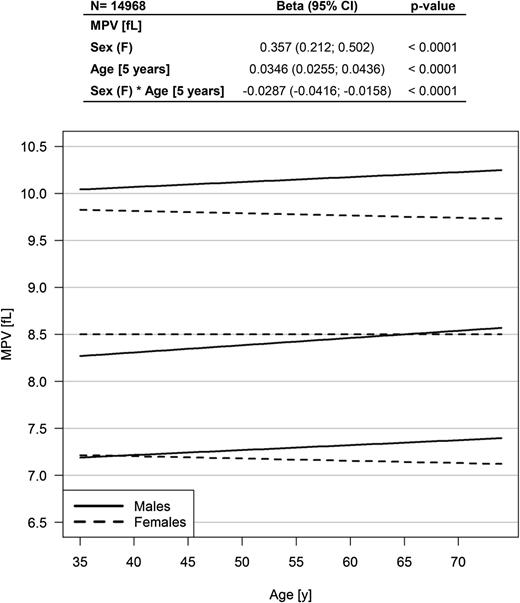
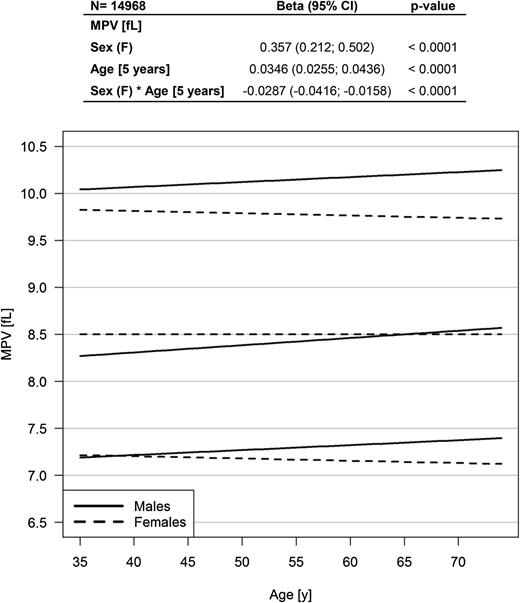
Of the 15 010 participants enrolled at baseline in the GHS cohort, MPV was available for 99.7% (n = 14 968). The age range of the study sample at the time of MPV measurement was 35 to 74 years with a mean of 55 years, and 49.4% were female. Linear regression analysis presented a higher MPV in females compared with males with an estimated β of 0.357 (95% CI, 0.212 to 0.502; P < .0001; Figure 2); however, there was a significant interaction effect between sex and age. The graphic in Figure 2 displays an increase in MPV in males only, with higher MPV in females until the age of approximately 65 years with an inversion of the relationship at older ages. Females in the reference group had an MPV comparable to that of females in the population sample (8.5 fL [95% CI, 8.0 to 9.1 fL] vs 8.5 fL [95% CI, 8.0 to 9.0 fL]; P = .2). Males from the reference group showed significantly lower MPV compared with males in the population sample (8.3 fL [95% CI, 7.8 to 8.8 fL] vs 8.4 fL [95% CI, 7.9 to 9.1 fL]; P < .0001).
Distribution of MPV in males and females in the population sample (N = 14 968). Effects of sex and age on MPV by using quantile regression for the median. The graphic shows the median values of MPV (middle lines) with 5th and 95th percentile (bottom and top lines) for males (solid lines) and females (dashed lines).
Distribution of MPV in males and females in the population sample (N = 14 968). Effects of sex and age on MPV by using quantile regression for the median. The graphic shows the median values of MPV (middle lines) with 5th and 95th percentile (bottom and top lines) for males (solid lines) and females (dashed lines).
Genetic variation and MPV
Supplemental Table 1 presents the SNPs evaluated in this study. By using genotype linear association analysis, we tested their association with MPV. For 19 of these SNPs, which were not on the Affymetrix array used in this study, an SNP in linkage equilibrium on the array was chosen with r2 > .8 between the lead SNP and tag SNP. By correlation analysis, we identified 14 SNPs that were strongly correlated with each other (Pearson correlation coefficient R2 ∼1) as shown in supplemental Figure 4. We consequently excluded 7 of these SNPs from multivariable linear regression analysis.
Linear regression analysis adjusted for age revealed 7 SNPs for females and 4 SNPs for males associated with higher MPV (Table 3). Three of these SNPs (rs342293, rs7961894, and rs342251) were associated with MPV in both sexes. A multivariable model that included both genetic and nongenetic factors by using the variable stepwise selection by Akaike information criterion explained 20.4% of the MPV variance in females and 18.6% in males (Table 4). Compared with the sum of all nongenetic factors, genetic factors explained 8.6% of MPV variance in females and 6.8% in males. WBC count and fibrinogen concentrations remained independently positively correlated with MPV in both males and females (P < .001), whereas platelet count persisted as being negatively correlated (P < .001). Oral contraceptives were still associated with higher MPV in females with an estimated β of 0.271 (95% CI, 0.109 to 0.433; P = .001), whereas smoking correlated with higher MPV in males (β, 0.103; 95% CI, 0.0094 to 0.196; P = .031). Of 7 SNPs initially associated with MPV in females, 6 remained independently associated with higher MPV, and of 4 SNPs in males, 2 remained as determinants in multivariable linear regression analysis.
MPV, SNP, and CVRF interaction
To evaluate effects on MPV of interaction between SNPs and CVRFs, we performed multivariable linear regression analysis that included the associated SNP and an interaction term with each CVRF as a separate variable (supplemental Table 2). Interestingly, the rs649729 variation in females was associated with an MPV increase of 0.145 fL (P = .023), and the presence of this SNP variation together with smoking was associated with a further increase in MPV of 0.251 fL (P = .019). An rs4774471 variation was associated with a significant increase in MPV of 0.197 fL in combination with hypertension in females (P = .021). In males, there was a significant interaction between a family history of MI or stroke and rs10506328 and/or rs2296828 variation leading to MPV increases of 0.194 fL (P = .042) and 0.213 fL (P = .024), respectively.
MPV and total mortality
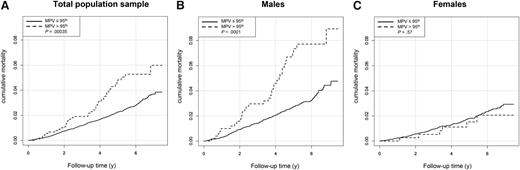
A total of 365 deaths were registered during the follow-up period up to December 2014 with a median follow-up time of 5.0 years (interquartile range, 3.6-6.3 years). As shown in Figure 3A, the cumulative mortality in individuals with MPV above the reference limit (>10.1 fL) was significantly worse (P = .00035) compared with individuals with MPV below the 95th percentile. Analysis of cumulative mortality, stratified for sex, showed a significant difference for males only (Figure 3B-C). Cox regression analysis adjusted for age and sex revealed that an increase in MPV of 1 fL was associated with an increase of 16% in mortality (hazard ratio, 1.16; 95% CI, 1.04 to 1.29; P = .0063). In addition, adjusting for traditional CVRFs and oral contraceptives did not significantly change the association between higher MPV and increased mortality (supplemental Table 3A). Only after further adjusting the Cox regression analysis for cardiovascular diseases was the significant association between MPV and mortality lost (supplemental Table 3B).
Total mortality and MPV. Plots of cumulative mortality depending on MPV in (A) the overall sample (N = 14 576), (B) in males (n = 7,319), and (C) in females (n = 7,257). Individuals were stratified for the 95th percentile of MPV in the reference group as cutoff value (cutoff values for MPV: overall sample, 10.1fL; males, 9.96 fL; females, 10.1 fL).
Total mortality and MPV. Plots of cumulative mortality depending on MPV in (A) the overall sample (N = 14 576), (B) in males (n = 7,319), and (C) in females (n = 7,257). Individuals were stratified for the 95th percentile of MPV in the reference group as cutoff value (cutoff values for MPV: overall sample, 10.1fL; males, 9.96 fL; females, 10.1 fL).
Discussion
This is the first sex-stratified comprehensive analysis of several genetic and nongenetic factors on MPV in a population-based cohort. Interestingly, despite commonalities, the importance of this approach is highlighted by substantial differences in the profiles of genetic and nongenetic determinants for MPV between males and females.
This is the first study of an adult population-based sample that investigates MPV and total mortality in an analysis stratified by sex. In non-sex-specific analyses, higher MPV has already been associated with total mortality in the general population and with vascular mortality in a large population of hospitalized patients.12,16 We now show that an MPV outside the reference range is associated with a higher risk of death in males only. Cox regression analysis demonstrated that MPV is a robust determinant of increased mortality after adjusting for age, sex, oral contraceptive use, and traditional CVRFs. The loss of association between MPV and mortality after adjusting for heart failure, peripheral arterial disease, and stroke, which have been associated with an increased MPV,27-29 suggests a causal relationship between MPV and the development of cardiovascular disease or vice versa, which needs to be further explored.
Second, analysis displayed another interaction between sex and age without an effect of age on MPV in women. As also observed by others, MPV was significantly higher in females compared with males and it increased with age in the total sample.14,16,19,30 However, the study implies that MPV was higher in females until the age of approximately 65 years, after which MPV was higher in males because of the absent relation with age in women.
Third, this study confirmed the influence of female hormonal status on MPV, particularly in the presence of menstruation or intake of oral contraceptives. Female sex hormones have been suggested to have an effect on the regulation of platelet indices, because the role of endogenous estrogen on proplatelet formation has been demonstrated in animal models.31 Estrogen replacement therapy has also been associated with an increase in MPV in postmenopausal women; however, we could not confirm an independent relationship in our study.32 A recent study reported an increased platelet count in menstruating women suggesting that loss of iron during bleeding could stimulate platelet production.33-35 Higher MPV in menstruating women can be explained by the increased platelet production with release of young platelets, which are known to have larger volume and reactivity compared with mature platelets.36
Fourth, MPV was significantly and positively associated with CVRFs in males, in particular with smoking, hypertension, and high glucose levels. Smoking has been previously associated with increased MPV, and studies have demonstrated that duration and intensity of active and/or passive smoking influences MPV.12,37 Furthermore, smoking cessation was associated with decreased MPV values.38 Higher MPV in smokers could partly be induced by smoking-mediated inflammation, as demonstrated by the independent correlation between highly sensitive CRP and smoking.39 Our study confirmed this finding and also displays smoking as being associated with higher MPV independently of WBC count, CRP, and fibrinogen. We also confirmed the association between MPV and WBC count and/or fibrinogen, which supports the link between platelets and inflammation in both males and females.19,40,41
Fifth, this is the first study to include all known genetic variants from large GWASs in exploring MPV heterogeneity at the population level. Genetic variants associated with MPV explained 8.6% of the MPV variation in females and 6.8% in males. In a multivariable linear regression analysis, 6 SNPs in females and 2 SNPs in males remained independently positively associated with MPV. In females, we determined an increase in MPV of 0.251 fL for an interaction of the rs649729 polymorphism (which was correlated with MPV only in females) with smoking. Located on chromosome 2p23.1, this intronic SNP lies in the Eps15 homology (EH) –domain containing 3 (EHD3) gene which is highly expressed in brain and heart and functionally associated with regulation of intracellular protein transport, membrane trafficking, and endocytosis.42 Recently, the EHD3 polymorphisms, including rs649729, have been associated with major depressive disorder in females only.43 This mechanism may explain the recent finding of higher MPV in patients with major depression.44 Another interaction in females was found between hypertension and rs4774471. This SNP is associated with the TPM1 gene on chromosome 15q22.2 that encodes tropomyosin I, which regulates the calcium-dependent interaction of actin and myosin, a key step in platelet formation.25 rs4774471 was associated with higher MPV in both sexes. In males, we observed an important interaction between 2 SNPs, rs10506328 (nuclear factor erythroid 2 [NFE2] and coatomer protein complex ζ1 [COPZ1]) and rs2296828 (dedicator of cytokinesis [DOCK8]), and family history of MI and/or stroke that was associated with higher MPV. rs10506328 (located on chromosome 12q13.13) has been associated with the NFE2 gene which is critical for megakaryocyte development and proplatelet formation, whereas the COPZ1 gene, which encodes a subunit of coatomer protein complex 1 (COPI), is involved in intracellular traffic and autophagy.45,46 The DOCK8 gene on chromosome 9p24.3 (rs2296828) regulates interstitial dendritic cell migration and is critical for the survival and function of natural killer T cells.47 Family history of MI and/or stroke is known to be a major cardiovascular risk factor. The presence of these two SNPs identified in our study might represent an additional factor of increased thrombotic risk.
Finally, antithrombotics, aspirin, and diuretics in both males and females and antilipemic and antihypertensive drugs in males were consistent with an altered MPV when using univariate models adjusted for age. However, the effects lost significance after analyzing them in multivariable linear regression. In the literature, statins were described as decreasing MPV irrespective of their cholesterol-lowering effect.48,49 Amlodipine-based therapy has been associated with a decrease in MPV, whereas low-dose aspirin showed no effect on MPV in healthy volunteers after 7 days of ingestion of 81 mg aspirin.7,50 However, effects of medical treatment on MPV were beyond the scope of this study, and other study designs and/or study samples are necessary for investigating this interesting issue in more detail.
The variability of MPV as a result of changing preanalytics, however, is a major limiting factor in using this parameter as a marker of platelet reactivity in clinical practice. Time to measurement after blood sampling, storage temperature of sample tubes, type of anticoagulant used, and the instrument used for MPV measurements have all been implicated in MPV variation and discordance among studies.51 Because platelets shrink in citrate and swell in EDTA, several groups proposed different measuring times ranging from within 1 hour regardless of the anticoagulant used to 120 minutes for EDTA and 60 minutes for citrate anticoagulant.22,52 A recent study by Shah et al50 also observed platelet swelling in EDTA until 180 minutes after blood withdrawal. Interestingly, Diaz et al53 reported a progressive increase in MPV with storage in EDTA measured at different time intervals up to 24 hours. To control for variation in these preanalytical factors, we performed our analysis under standardized conditions measuring MPV between 30 and 90 minutes after blood sampling. Other strengths of this study are the large sample size and the highly standardized setting that supported the comprehensive analysis of MPV with both genetic and nongenetic determinants in a sex-specific approach.
In conclusion, this study provides evidence for substantial heterogeneity in the profile of determinants for MPV between sexes. To the best of our knowledge, this is the first large population-based study showing evidence of interaction between genetic variations, CVRFs, and MPV, suggesting a need for deeper insight into the interplay between environmental factors and genetic variability for MPV as a biomarker that indicates platelet function. Additional studies should investigate the role of MPV in males for increased risk of thrombosis and development of cardiovascular disease, because they are significantly determined by traditional CVRFs and associated with total mortality.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by contract AZ 961-386261/733 from the government of Rhineland-Palatinate (Stiftung Rheinland-Pfalz für Innovation,), by research programs Wissen schafft Zukunft and Center for Translational Vascular Biology of the Johannes Gutenberg-University of Mainz, and contracts with Boehringer Ingelheim and Philips Medical Systems, including an unrestricted grant for the Gutenberg Health Study. P.S.W., S.B., T.Z., and T.M. are principal investigators of the German Center for Cardiovascular Research. H.t.C. is a Fellow of the Gutenberg Research Foundation.
Authorship
Contribution: M.P.-N. designed and performed research, analyzed and interpreted data, and wrote the paper; A.S. performed the statistical analysis; M.I.H. and V.G. contributed to discussion of the results and to critical review; D.L.-R. collected laboratory and clinical data; H.M.H.S., H.t.C., K.J.L., and T.M. contributed to writing the paper and to critical review; E.P., H.B., M.B., N.P., S.B., and T.Z. performed research; P.S.W. designed and performed research, interpreted data, and contributed to writing the paper; and all authors have read and approved the manuscript in its current form.
Conflict-of-interest disclosure: M.I.H., V.G., and P.S.W. were funded by the Federal Ministry of Education and Research (BMBF 01EO1003). H.t.C. is a Fellow of the Gutenberg Forschungskolleg of the Johannes Gutenberg-University, Mainz, Germany. P.S.W. has received research funding from Boehringer Ingelheim; Philips Medical Systems; sanofi-aventis; Bayer Vital; Daiichi Sankyo Europe; Institute for the Modernization of Economic Base and Employment Structures; Portavita; Federal Institute for Occupational Safety and Health; Health Economy Initiative, Ministry of Health, and Ministry of Economics, Rhineland-Palatinate; Federal Ministry of Education and Research; Federal Ministry of Health, Rhineland-Palatinate; and Mainz Heart Foundation, and has received honoraria for lectures or consulting from Boehringer Ingelheim and Public Health, Heinrich-Heine-University Düsseldorf. The remaining authors declare no competing financial interests.
Correspondence: Marina Panova-Noeva, Clinical Epidemiology, Center for Thrombosis and Hemostasis, Universitätsmedizin der Johannes Gutenberg-Universität Mainz, Langenbeckstrasse 1, 55131 Mainz, Germany; e-mail: marina.panova-noeva@unimedizin-mainz.de.