Abstract
Allogeneic stem cell transplantation (SCT) is a unique procedure, primarily in patients with hematopoietic malignancies, involving chemoradiotherapy followed by the introduction of donor hematopoietic and immune cells into an inflamed and lymphopenic environment. Interruption of the process by which recipient alloantigen is presented to donor T cells to generate graft-versus-host disease (GVHD) represents an attractive therapeutic strategy to prevent morbidity and mortality after SCT and has been increasingly studied in the last 15 years. However, the immune activation resulting in GVHD has no physiological equivalent in nature; alloantigen is ubiquitous, persists indefinitely, and can be presented by multiple cell types at numerous sites, often on incompatible major histocompatibility complex, and occurs in the context of intense inflammation early after SCT. The recognition that alloantigen presentation is also critical to the development of immunological tolerance via both deletional and regulatory mechanisms further adds to this complexity. Finally, GVHD itself appears capable of inhibiting the presentation of microbiological antigens by donor dendritic cells late after SCT that is mandatory for the establishment of effective pathogen-specific immunity. Here, we review our current understanding of alloantigen, its presentation by various antigen-presenting cells, subsequent recognition by donor T cells, and the potential of therapeutic strategies interrupting this disease-initiating process to modify transplant outcome.
Introduction
Allogeneic hematopoietic stem cell transplantation (SCT) remains an important curative therapy for hematological malignancies. The therapeutic graft-versus-leukemia (GVL) effect is immunological in nature and mediated by donor T and natural killer cells recognizing recipient allogeneic, hematopoietic or leukemia-specific antigens.1 GVL is thus associated with pathogenic immune responses against non-hematopoietic tissue that manifest as graft-versus-host disease (GVHD), the major procedural limitation. GVHD presents principally as acute and chronic diseases that are characterized by tissue apoptosis and fibrosis, respectively. The 2 processes may also present simultaneously in various overlap syndromes late after transplant. Critically, 15% to 20% of SCT recipients will develop severe GVHD that is refractory to therapy and die.1,2 Current prevention and treatment of GVHD rely on the broad suppression of T cells and inflammation with calcineurin inhibitors and corticosteroids, respectively, which also impacts leukemia and pathogen-specific immunity. New approaches include posttransplant cyclophosphamide and alternative immune suppression that include rapamycin. There is now an ever-increasing use of alternative (as opposed to major histocompatibility complex [MHC]-matched sibling) donors for SCT. Transplants using these donors, particularly those that are HLA mismatched such as umbilical cord or haploidentical (ie, fully MHC mismatched), increase the risk of life-threatening GVHD and the necessity for intensive therapeutic interventions involving various forms of T-cell depletion.3,4 Concurrently, the use of reduced-intensity and nonablative conditioning has increased to allow the transplantation of increasingly older patients,5 and these procedures rely heavily on immunological GVL effects for their therapeutic efficacy.
It is clear that the targets of GVHD are MHCs and peptides presented therein (in MHC-mismatched donors and recipients) and/or minor histocompatibility antigens (mHAs) in both MHC-mismatched and MHC-matched donor–recipient pairs. There has, however, been less clarity in regard to the nature of alloantigen presentation responsible for the initiation of immune responses in donor T cells that lead to GVHD and, in particular, the cells and molecular pathways involved. This review outlines recent developments in the field that improve our understanding of alloantigen presentation initiating pathological and protective immunity after SCT, highlighting a complex but highly modifiable process.
Alloantigen
When considering allogeneic SCT in which the donor and recipient are MHC matched, the alloantigens that initiate GVHD are defined as mHAs. Broadly, these mHAs are peptides generated by polymorphic genes that differ between donor and host that can in turn be presented by MHCs. Most of the mHAs defined to date are presented in HLA class I,6,7 predominantly due to the molecular techniques used for their identification. There is increasing recent enthusiasm for identification of hematopoietic-restricted mHAs, because these antigens are attractive for use in immunotherapy trials to augment GVL8 and prevent relapse (reviewed in Bleakley et al9). Interestingly, the continued expression of mHAs on nonhematopoietic cells appears to result in alloreactive T-cell exhaustion and impaired GVL effects.10,11 Thus, the identification of hematopoietic-restricted mHAs is highly desirable, and a number of such mHAs have been identified, including HA-1, HA-2, LRH-1, and ACC-2 (reviewed in Bleakley et al9). As outlined below, self-peptides (and thus not mHAs) may also be seen as foreign by donor T cells when presented in a mismatched MHC.
Recognition of alloantigen by donor T cells
The depletion of donor T cells results in a low incidence of acute and chronic GVHD, albeit at the expense of leukemia relapse.12,13 Likewise, T-cell–replete autologous SCT is associated with high relapse rates relative to allogeneic SCT, suggesting T-cell stimulation by alloantigen is critical for both GVHD and GVL effects.14 GVHD can be conceptualized as MHC class I and/or MHC class II dependent (ie, CD8+ and CD4+ T cells, respectively). Mismatches at HLA class I and II are significant risk factors for severe GVHD and transplant-related mortality3 ; thus, both CD8+ T cells and CD4+ T cells are involved, and indeed the depletion of either T-cell subset is insufficient to prevent GVHD.15,16 It is important to note that mHAs are also presented and recognized within MHC class I and II, and so both CD4 and CD8 T cells are also involved in GVHD after MHC-matched SCT. However, the origin of the antigen and the process of presentation differ between the 2 pathways, and consideration of this is critical to the understanding GVHD as a disease process. In considering antigen presentation and T-cell recognition, it is also important to consider MHC-matched and MHC-mismatched SCT separately.
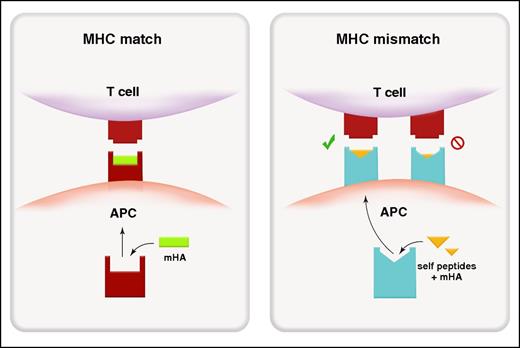
In MHC-matched SCT, alloantigens presented in MHC class I are predominantly endogenous in origin (ie, the antigen is intrinsic to the cell),17 and donor T cells recognize, via the T-cell receptor (TCR), polymorphic recipient peptides presented within a MHC that is shared by both the donor and recipient (Figure 1). In regard to the process of presentation, self- or viral cytosolic proteins are processed within the proteosome and then transported into the endoplasmic reticulum by the transporter associated with antigen processing (TAP). There, the endoplasmic reticulum aminopeptidases trim peptides to 8 to 10 amino acids for loading into MHC class I before transfer to the surface (reviewed in Blum et al18). Exogenous antigens (ie, the antigen is extrinsic to the cell) can also be presented within MHC-class I by a process termed cross-presentation and is principally thought to occur in sub-specialized dendritic cell (DC) subsets (CD8+ and/or CD103+ in mouse, BDCA3+ in humans). Here, phagocytosed exogenous antigen is translocated into the proteosome for processing within MHC class I. The importance of cross presentation to GVHD pathology remains unclear at this point although it is clearly a highly active process19 that is predicted to be critical in the generation of pathogen-specific immunity.
Alloantigen presentation and recognition in MHC-matched and MHC-mismatched transplantation. Peptide recognition in MHC-matched transplant (left). Because donor T cells are selected through positive and negative selection in donor thymus, the donor TCR recognizes host-derived (non-self) mHAs (green) within an MHC (red) that is shared between donor and host. Molecular mimicry in MHC-mismatched transplantation (right). The donor TCR can recognize a mismatched host MHC (blue) loaded predominantly with antigenic peptide derived from ubiquitous self-proteins (yellow) rather than polymorphic mHAs.
Alloantigen presentation and recognition in MHC-matched and MHC-mismatched transplantation. Peptide recognition in MHC-matched transplant (left). Because donor T cells are selected through positive and negative selection in donor thymus, the donor TCR recognizes host-derived (non-self) mHAs (green) within an MHC (red) that is shared between donor and host. Molecular mimicry in MHC-mismatched transplantation (right). The donor TCR can recognize a mismatched host MHC (blue) loaded predominantly with antigenic peptide derived from ubiquitous self-proteins (yellow) rather than polymorphic mHAs.
In contrast to MHC class I, alloantigens presented in MHC class II are predominantly exogenous in origin, and donor T-cells again recognize, via the TCR, polymorphic recipient peptides presented within MHC that are shared by both the donor and recipient. Thus, alloantigen may be presented in MHC-class II by recipient or donor antigen-presenting cells (APCs). Exogenous antigen is acquired by APC via phagocytosis of dead or necrotic cells, endocytosis, or macropinocytosis. The former 2 processes are receptor mediated (eg, clathrin), and these pathways exist to various levels of efficiency in all professional APCs (ie, B cells, monocytes/macrophages, and DCs). Exogenous proteins are processed in the lysosome and transported to the endosome for loading into MHCs. MHC class II molecules themselves are transported from the endoplasmic reticulum to the Golgi as a complex with an invariant chain that is then processed in the late endosome by HLA-DM to release the class II–related invariant chain peptide from the MHC, facilitating replacement by peptide of appropriate affinity (reviewed in Blum et al18 ) before transfer to the cell surface. Endogenous antigens can also be presented directly within MHC class II during periods of cellular stress in a process known as autophagy. In this process, endogenous proteins of nuclear, mitochondrial and cytoplasmic origin are incorporated into autophagosomes, which then fuse with the lysosome to allow antigen delivery into the MHC class II pathway. Although it has recently been demonstrated that autophagy-deficient recipient DCs paradoxically induce more GVHD than wild-type DCs,20 it is currently unclear whether this relates to effects on antigen presentation per se. Thus, the relative contribution of autophagy to antigen presentation within MHC class II after SCT remains unknown at this point, but given the inflammation and cellular stress therein, the process itself is likely highly relevant.
A third “semi-direct” pathway of antigen presentation has recently also been described. Here, donor cells can acquire recipient cell-derived cell-surface membrane including MHC class I and class II via either trogocytosis or exosome uptake.19,21 In this process, MHC molecules loaded with alloantigen are transferred from neighboring cells in a cell contact or an exosome-secretion–dependent manner, which may subsequently activate donor T cells.22 This process is reported to contribute to T-cell activation or suppression22,23 and is likely important as a means of antigen acquisition and, potentially, presentation.19,21
In transplant settings where MHC mismatches are present, donor T cells react to recipient APCs at a very high frequency (ie, 1% to 10%).24 It is now clear that in this setting, donor T cells can cross-react to non–self (host)-MHCs loaded with an antigenic peptide in a process known as molecular mimicry.24-27 Thus, in MHC-mismatched transplants, recipient MHC molecules loaded with various self-peptides (ie, peptides that may not be foreign or allogeneic to the donor) may present a conformational footprint that allows engagement of a donor TCR (Figure 1). As an example, HLA-B*4402 and HLA*4405 presenting self-peptides such as ATP-associated proteins can stimulate a TCR clone that recognizes a viral peptide presented within HLA-B*0801 (its self-MHC).27 Thus, there would appear an extensive capacity for conformational changes in the MHC–peptide complex to be recognized by MHC-disparate T cells. In addition, the TCR itself also appears capable of undergoing conformational “fine-tuning” to accommodate minor conformational alterations in MHC-peptide complexes (reviewed in Gras et al28 ). Presumably, these mechanisms are responsible for the severe GVHD risk when transplanting across multiple MHC mismatches relative to a single-locus mismatch.29,30 In the former situation, the high number of mismatched loci increases the probability of molecular mimicry of peptide–MHC complexes for numerous T-cell clones. Indeed, this principal was elegantly demonstrated in 1986 by Sprent et al.31 By using B6 bone marrow transplantation (BMT) recipients with a single mutation in MHC class I or class II (H2-Kbm1 or H2-Ab1bm12), they demonstrated the ability of B6 donor CD8 and CD4 T cells respectively to induce lethal GVHD, despite the complete absence of any mHA disparities between donor and host, consistent the ability of recipient “self”-peptides to be recognized in the context of MHC disparity. This scenario of course predicts that unlike MHC-matched SCT, identification of mHAs may be relatively fruitless in MHC-mismatched SCT.
The requirement for host and/or donor APCs for the initiation and maintenance of GVHD has been the subject of considerable study. Historically, this has been referred to as direct or indirect antigen presentation. In direct antigen presentation, donor T cells recognize MHC–peptide complexes on host APCs; in indirect antigen presentation, donor T cells recognize recipient-derived peptides loaded in the MHC of donor APCs.32,33 A seminal study by Shlomchik et al demonstrated that host APCs are necessary and sufficient for the initiation of lethal acute GVHD within MHC class I.17 Subsequent studies confirmed the same held true for MHC class II.34,35 However, the nature of the APCs required appears to differ within MHC class I and II. Within MHC class I, hematopoietic-derived recipient APCs appear to be critical,17,36 although nonhematopoietic APCs may play a minor role.36 Within MHC class II, both hematopoietic and nonhematopoietic APCs can initiate lethal acute GVHD, and nonhematopoietic APCs appear dominant.35 In contrast, donor APCs appear inefficient at inducing GVHD in isolation of recipient APCs regardless of the pathway.35,37
Recipient APC subsets
As previously mentioned, host APCs have been shown to be important for the induction of GVHD. Subsequently, many studies have attempted to identify the critical APC subsets involved, predicated on the notion that deletion may prevent GVHD. Longstanding dogma has held that recipient DCs are the APCs responsible for GVHD, based on their ability to initiate disease when transferred into MHC-deficient recipients38 and their transient persistence and activation after total body irradiation (TBI).39 In similar studies, host plasmacytoid DCs could also initiate GVHD in lethally irradiated recipients, whereas host B cells could not.40 In relation to the effects of TBI and myeloablative conditioning, the incidence of acute GVHD is still high, albeit delayed, after reduced-intensity conditioning in the clinic. Thus any beneficial effects of reduced inflammation in this setting is likely countered by the persistence of recipient APC and subsequent initiation of GVHD at later time points after transplant. In this regard, conditioning intensity, inflammation and the turnover of APC after clinical BMT is likely to be crucial in determining the relative importance of various APC subsets (ie, donor vs host; hematopoietic vs non-hematopoietic) in the initiation and maintenance of GVHD. Certainly DC in skin are predominantly donor in origin by at least 40 days after BMT and this is accelerated by GVHD after reduced-intensity but not myeloablative conditioning.41 DCs in lymphoid tissue turnover within two weeks in mice42 but clear data defining this in humans is lacking. In contrast to DC, macrophage turnover in humans in tissue is considerably slower than DC and may not be complete even by one year.41 Together, current data suggests that host DC are short lived and donor DC are likely to predominate in relevant lymphoid organs at the time of engraftment in patients receiving ablative conditioning.
In relation to DC and GVHD, preclinical studies have established the ability of recipient DC to induce GVHD in recipients otherwise devoid of all functional APC but not their importance in recipients where all APC are competent. Recently, a new generation of transgenic mice in which CD11c+ cells can be conditionally deleted has turned this concept on its head, demonstrating that recipient DC are not required to initiate MHC-class II-dependent GVHD and may actually regulate disease with the induction of T cell apoptosis.35,43,44 Furthermore, recipient plasmacytoid DCs, B cells, macrophages, and Langerhans cells also appear to be redundant in isolation for the initiation of lethal acute GVHD and capable of regulating disease.35,43,45-48 The pathways involved in regulation by these professional APC subsets involve interleukin-10 (IL-10) for B cells,49 CD47 and donor T-cell uptake for macrophages,45 and clonal deletion for DCs.35 In relation to DCs, it may not be surprising that the APC subset specialized for the most efficient presentation of limiting amounts of antigen act to delete T cells when antigen is ubiquitous and present in excess early after SCT following chemoradiotherapy. Nevertheless, these findings raise the possibility that considerable redundancy exists within recipient professional APCs in relation to their ability to induce GVHD, that vanishing small numbers are capable of initiating severe GVHD, or alternatively that an additional, likely tissue-residing APC not depleted by the above approaches is capable of inducing GVHD.
In this vein, recipient nonhematopoietic APCs, once activated by conditioning chemoradiotherapy, have emerged as APCs that are highly efficient in initiating MHC class II–dependent GVHD.35,36 Indeed, TBI induces the expression of molecules required for costimulation (CD40, CD80, and CD86) on nonhematopoietic MHC class II+ cells.35 To date, fibroblasts have been shown to be capable of inducing cytotoxic T-lymphocyte responses,50-52 as have epithelial cells once induced to express MHC class II by inflammation (particularly interferon-γ; Figure 2).53-55 This concept is supported by preclinical studies whereby allogeneic nonhematopoietic, but not hematopoietic, APCs were required for acute GVHD lethality56 and biopsy samples from patients demonstrating high expression of HLA class II on colonic nonhematopoietic cells after transplantation.57 Recently, it has been demonstrated that a HLA-DP1 variant (rs9277534G), which results in higher protein expression than the variant rs9277534A, is associated with an increased incidence of grade II-IV GVHD, suggesting that the levels of HLA class II expression per se correlate with the severity of GVHD.58 Intriguingly, consistent with CD4+ T-cell–dependent GVHD, the depletion of host DCs has no effect and may also augment MHC class I–dependent GVHD.43 Thus, multiple independent studies, in multiple models, have demonstrated that recipient DCs are not responsible for the initiation of GVHD and, moreover, that their deletion is likely to be detrimental.
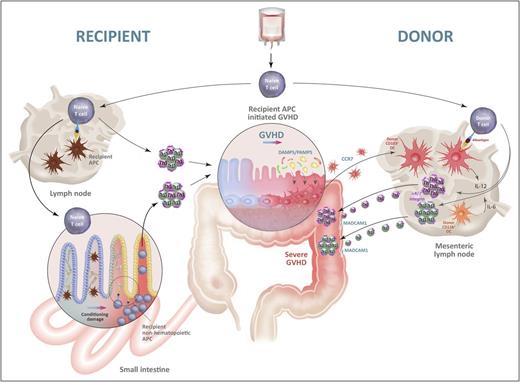
The initiation and amplification of GVHD by alloantigen presentation. Naive donor T cells contaminating the stem cell graft migrate into recipient lymph nodes and/or GVHD target organs, particularly the gastrointestinal (GI) tract. They encounter recipient alloantigen presented by recipient APCs in the lymph nodes and/or by recipient nonhematopoietic APCs, including those in target tissue (eg, fibroblasts, epithelial cells). The latter obtain functional antigen presentation capacity following tissue damage induced by chemoradiotherapy during conditioning and cytokines generated following donor T cell activation. Hereafter, donor T cells proliferate and differentiate into Th1 and Th17 cells and induce GVHD in the target tissues, particularly the GI tract. Tissue damage within the colon at this point allows microbiome-derived damage-associated molecular pattern/pathogen-associated molecular pattern (DAMP/PAMP) signals to expand and activate donor CD103+ DCs in situ, which subsequently migrate into the mesenteric lymph node, under the influence of CCR7. Within the mesenteric lymph node, these donor DCs secrete IL-12 and present alloantigen to donor T cells to further drive pathological Th1 and Th17 differentiation while imprinting gut-homing α4β7 integrins, permissive of massive secondary migration into the gut, resulting in fulminant disease. Schematic highly modified from Koyama et al42 with permission.
The initiation and amplification of GVHD by alloantigen presentation. Naive donor T cells contaminating the stem cell graft migrate into recipient lymph nodes and/or GVHD target organs, particularly the gastrointestinal (GI) tract. They encounter recipient alloantigen presented by recipient APCs in the lymph nodes and/or by recipient nonhematopoietic APCs, including those in target tissue (eg, fibroblasts, epithelial cells). The latter obtain functional antigen presentation capacity following tissue damage induced by chemoradiotherapy during conditioning and cytokines generated following donor T cell activation. Hereafter, donor T cells proliferate and differentiate into Th1 and Th17 cells and induce GVHD in the target tissues, particularly the GI tract. Tissue damage within the colon at this point allows microbiome-derived damage-associated molecular pattern/pathogen-associated molecular pattern (DAMP/PAMP) signals to expand and activate donor CD103+ DCs in situ, which subsequently migrate into the mesenteric lymph node, under the influence of CCR7. Within the mesenteric lymph node, these donor DCs secrete IL-12 and present alloantigen to donor T cells to further drive pathological Th1 and Th17 differentiation while imprinting gut-homing α4β7 integrins, permissive of massive secondary migration into the gut, resulting in fulminant disease. Schematic highly modified from Koyama et al42 with permission.
The GI tract, danger signals, and acute GVHD
The primacy of the gastrointestinal tract in severe, life-threatening acute GVHD is well established.59 The gastrointestinal tract and the colon in particular contain an extensive microbiome60 and as such act as a reservoir for DAMP/PAMP signals, particularly in the context of high-dose chemoradiotherapy.42 In clinical transplantation, antibiotic decontamination, particularly in regard to anaerobe eradication, can reduce the incidence of acute GVHD.61,62 However, recent data suggest that some anaerobe species are in fact highly protective from GVHD,63 and so current studies focus on qualitative rather than quantitative characteristics of the microbiome in the gastrointestinal (GI) tract. Experimental models have been used to address the importance of DAMP/PAMP-mediated activation of host and/or donor APCs, including effects on antigen presentation. Although C-type lectin receptors on residual host macrophages that recognize fungal components are important in pulmonary GVHD in particular,64 surprisingly, neither MyD88 and TRIF signals, which mediate Toll-like receptor (TLR) signals, nor type I interferon signals in recipient cells enhanced GVHD.65 On the other hand, it is well established that TLR signaling of donor cells exacerbates acute GVHD.66 Recently, we demonstrated that donor CD103+ DCs that migrate from the colon to the mesenteric lymph nodes under the guidance of CCR7 following activation and expansion in response to TLR (MyD88 and TRIF) or receptor for advanced glycation end products (RAGE) signals are critical in promoting GVHD lethality.42 Importantly, this effect is dependent on the presence of GVHD initiated by recipient APCs that in turn generates a profound feed-forward loop of antigen presentation by donor DCs with subsequent pathological T-cell differentiation (Th1/Th17) and (α4β7 integrin–dependent) migration to the GI tract (Figure 2).
Cytokines and acute GVHD
It is clear that allogeneic disparity induces a T-cell cascade culminating in organ-specific damage (GI tract, liver, and skin) that manifests as acute GVHD. In the first 14 days of transplant, hyperacute GVHD often occurs in conjunction with systemic symptoms including fever, weight gain, and pulmonary edema. This syndrome commonly occurs in heavily pretreated recipients receiving myeloablative conditioning and HLA-mismatched grafts.67 It is attractive to propose that this syndrome is a manifestation of cytokine dysregulation and akin to the cytokine release syndrome seen after infusion of chimeric antigen receptor T cells in which IL-6 appears to play a central role.68 Certainly, IL-6 is dysregulated after clinical transplantation,69 but the nature of the alloantigen presentation driving this syndrome remains unclear at this time.
Antigen presentation in chronic GVHD
Chronic GVHD is defined by fibrosis and scleroderma or bronchiolitis obliterans represent cardinal diagnostic manifestations. National Institutes of Health criteria describe classic chronic GVHD (without acute GVHD) and an overlap syndrome in which both acute and chronic GVHD appear together.70 Such complexity and diversity is difficult to model experimentally, although systems do exist where fibrosis is a feature.1,70,71 Although the role of antigen presentation in chronic GVHD remains less well understood than that in acute GVHD, emerging data suggest antigen presentation in the thymus and peripheral tissues plays an essential role in maintaining tolerance by promoting donor regulatory T-cell (Treg) homeostasis.72-74 MHC class II expression on donor DCs within the thymus is pivotal to control autoreactive (donor-reactive) thymic-emigrant T cells and the prevention of disease in animal models.72,75 Although the relevance of antigen presentation to clinical chronic GVHD remains less clear, thymic atrophy, immune suppression, and autoreactive T cells are certainly a cardinal feature of human chronic GVHD. Intriguingly, the transplantation of CD80/86-deficient or CD40-deficient donor bone marrow that lacks costimulatory signals for efficient MHC-class II antigen presentation does not cause this morbidity, concordant with previous findings in which thymic clonal deletion is functionally intact in these mice.76,77 Instead, these deficiencies result in protection from GVHD mediated by mature donor T cells within the graft.78 Donor DCs, both in the thymus and peripherally, are known to control Treg development and maintenance, respectively.79 Again, defects in Treg are also a cardinal feature of chronic GVHD, occurring within the context of CD4+ lymphopenia.74 This has led to the development of immune-restorative therapeutic strategies such as low-dose IL-2 to expand Treg.80,81 Antigen presentation by DCs to Treg is required for Treg homeostasis in general,82,83 although direct evidence for this after SCT has yet to be established. Donor B cells84,85 and macrophages86 have also been identified as mediators of chronic GVHD, although these effects would appear likely due to their effector function (in generating alloantibodies87 and TGFβ86 respectively) rather than antigen presentation per se. Again, exclusion of a role for antigen presentation requires further testing.
GVHD and the inhibition of antigen presentation
Although alloantigen presentation by donor DC in the GI tract is markedly enhanced early after SCT in the presence of acute GVHD,42 chronic GVHD is highly immunosuppressive, and indeed the major cause of mortality and morbidity in these patients is opportunistic infection. Although there are undoubtedly significant intrinsic T-cell defects during chronic GVHD, recent animal data have demonstrated defective antigen presentation, predominantly by donor DCs within MHC class II.88 Subsequent work demonstrates this results in overwhelming cytomegalovirus (CMV) disease with hepatic necrosis and lethality due to the inability to generate pathogen-specific immunity.89 Conversely, CMV reactivation may be correlated with reduced relapse of acute myeloid leukemia.90 Although CMV is detected in leukemic blasts91 and this is speculated to provide virus-derived antigens as GVL targets, the mechanisms involved will need further careful experimentation. Although the nature of the defect in antigen presentation (eg, self or allogeneic) within donor DC remains poorly defined, it appears to be the result of chronic inflammation and immune activation.
Antigen presentation and the separation of GVHD and GVL
Recipient hematopoietic APCs are crucial for the induction of both MHC class I– and class II–dependent GVL in MHC-matched mHA-mismatched models.92 Donor APCs are either not required37 or have limited capacity (relative to recipient APCs)92 to promote GVL, depending on the model systems used. Recently, the CD8+ DC subset has been suggested as a critical APC for the induction of GVL,93 although this appears somewhat at odds with data in relation to GVHD. On the other hand, MHC expression on leukemia cells is clearly critical for effective GVL responses. Thus, the expression of MHC class I on target cells is required for CD8+ T-cell–dependent GVHD and GVL, whereas MHC class II expression is required for CD4+ T-cell–dependent GVL, but not GVHD.94,95 The latter is due to the fact that inflammatory cytokines produced by CD4+ T cells can induce target tissue damage independent of cognate TCR–MHC interactions.34 Targeting presentation of not only mHA but also leukemia-associated antigens (eg, Wilms tumor antigen-1 or proteinase 3) are also promising approaches to enhance GVL effects without attendant risks of GVHD.96,97 Because donor APCs are minimally required, if at all, for GVL,37,92 transient interruption of alloantigen presentation by donor CD103+ DCs migrating from the colon would appear a cellular cascade central to the effective separation of GVHD and GVL. Antibodies with activity against molecules that are expressed on activated cells, including DCs,98 may be effective if they can be administered in the right window after SCT when donor DCs are active and recipient DCs have been eliminated. Of note, because donor APCs are likely crucial for the generation of pathogen-specific immunity, targeting donor DC or APC subsets will need to be studied with caution in the clinic. In addition, understanding the nature of and mechanisms by which recipient nonhematopoietic APCs function to initiate GVHD may be important keys to separating GVHD and GVL in the future. Finally, whether the defect in antigen presentation during chronic GVHD plays a role in the associated Treg deficiency remains to be elucidated but nonetheless serves as an attractive hypothesis.
Conclusions and future directions
Alloantigen presentation is central to both the therapeutic (GVL) and adverse effects (GVHD) of allogeneic SCT and as such represents a pathway of primary importance to clinical outcome. There would appear multiple therapeutic strategies worthy of exploration at this time, which are described in Table 1. Firstly, the adoptive transfer or induction of defined mHA-specific donor T cells9 as well as leukemia-associated antigen–specific T cells would appear an attractive immunotherapy approach to prevent and treat disease relapse. As long as the mHA is restricted to hematopoietic cells, donor vaccination could also be contemplated.8 Secondly, interruption of alloantigen presentation by donor DCs within the GI tract, at a time when recipient DCs have been eliminated, should separate GVHD effects and could be undertaken by inhibition of the chemokine signal (CCR7) required for migration of these DCs to primary lymphoid organs.42 The role of the microbiome in generating PAMPs that drive antigen presentation and cytokine secretion in the GI tract also makes it a highly attractive target for modulation after transplantation.99 It is important to note that many of our standard immune suppressants have also been reported to impact antigen presentation directly (Table 1; reviewed in Stenger et al100). Finally, a better understanding of the recipient nonhematopoietic APCs that induce GVHD and the pathways that are required for their pathogenicity, particularly autophagy, may allow for therapeutic intervention.35
Acknowledgments
The authors thank Madeleine Flynn of QIMR Berghofer for generation of the graphics. The authors apologize to the authors of the many important articles that could not be referenced due to space restrictions.
Authorship
Contribution: M.K. and G.R.H. wrote the manuscript.
Conflict-of-interest disclosure: G.R.H. has received funding from Roche for clinical studies of IL-6 inhibition. M.K. declares no competing financial interests.
Correspondence: Geoffrey R. Hill, Bone Marrow Transplantation Laboratory, QIMR Berghofer Medical Research Institute, 300 Herston Rd, Brisbane, QLD 4006, Australia; e-mail: geoff.hill@qimrberghofer.edu.au.