Key Points
TCM-derived CD19 CAR T–cell therapy is safe for treatment of poor-risk NHL patients undergoing autologous HSCT.
Addition of a CD28 costimulatory domain to the CAR, plus changes to T-cell product manufacturing, resulted in improved T-cell expansion.
Abstract
Myeloablative autologous hematopoietic stem cell transplantation (HSCT) is a mainstay of therapy for relapsed intermediate-grade B-cell non-Hodgkin lymphoma (NHL); however, relapse rates are high. In phase 1 studies designed to improve long-term remission rates, we administered adoptive T-cell immunotherapy after HSCT, using ex vivo–expanded autologous central memory–enriched T cells (TCM) transduced with lentivirus expressing CD19-specific chimeric antigen receptors (CARs). We present results from 2 safety/feasibility studies, NHL1 and NHL2, investigating different T-cell populations and CAR constructs. Engineered TCM-derived CD19 CAR T cells were infused 2 days after HSCT at doses of 25 to 200 × 106 in a single infusion. In NHL1, 8 patients safely received T-cell products engineered from enriched CD8+ TCM subsets, expressing a first-generation CD19 CAR containing only the CD3ζ endodomain (CD19R:ζ). Four of 8 patients (50%; 95% confidence interval [CI]: 16-84%) were progression free at both 1 and 2 years. In NHL2, 8 patients safely received T-cell products engineered from enriched CD4+ and CD8+ TCM subsets and expressing a second-generation CD19 CAR containing the CD28 and CD3ζ endodomains (CD19R:28ζ). Six of 8 patients (75%; 95% CI: 35-97%) were progression free at 1 year. The CD4+/CD8+ TCM-derived CD19 CAR T cells (NHL2) exhibited improvement in expansion; however, persistence was ≤28 days, similar to that seen by others using CD28 CARs. Neither cytokine release syndrome nor delayed hematopoietic engraftment was observed in either trial. These data demonstrate the safety and feasibility of CD19 CAR TCM therapy after HSCT. Trials were registered at www.clinicaltrials.gov as #NCT01318317 and #NCT01815749.
Introduction
For patients with diffuse large B-cell lymphoma (DLBCL) who have relapsed after initial multiagent chemotherapy, salvage chemotherapy followed by hematopoietic stem cell transplantation (HSCT) is the standard of care. Despite high-dose chemotherapy used to ablate the residual tumor, the 3-year progression-free survival (PFS) is only 39%.1 Among patients with mantle cell lymphoma (MCL), the 5-year PFS was 33% in 195 registry patients receiving HSCT.2 Transplantation performed in first complete remission (CR1) of MCL gives a 3-year PFS of 62% and provides the greatest survival advantage compared with either consolidation therapy at first remission3 or to HSCT performed in patients not in CR1.2 Major risk factors for relapse after HSCT for non-Hodgkin lymphoma (NHL) include persistent 18F-fluorodeoxyglucose–positron emission tomography (PET) positivity4-6 after salvage chemotherapy and adverse molecular genetics or histology. Because disease relapse or progression is the major cause of treatment failure following HSCT for NHL, we designed studies aimed at enhancing antitumor activity by incorporating adoptive cellular immunotherapy into the transplantation regimen.
T-cell products that are genetically engineered with chimeric antigen receptors (CARs) targeting CD19 have broad application for adoptive therapy of B-lineage malignancies and have recently shown tremendous potential in treatment of B-cell leukemia.7-10 CD19 CAR T–cell therapy response rates vary between the CD19+ malignancies, with overall response rates of up to 90% reported in acute lymphoblastic leukemia,11-13 with lower rates reported for lymphoma of 50% to 80% overall response rate.14-17 Because NHL has characteristics intermediate between leukemias and solid tumors, it is possible that a more prominent tumor immunosuppressive microenvironment in lymphoma could prevent antitumor T-cell proliferation, infiltration, and killing of tumors, thus contributing to the lower response rates to adoptive cellular immunotherapy. We hypothesized that, following HSCT, the immunosuppressive microenvironment would be diminished as a result of myeloablative conditioning and that administering CAR T–cell therapy during the hematopoietic reconstitution could eradicate posttransplant residual disease, leading to lower relapse rates after HSCT.18 A day 2 posttransplant CAR T–cell infusion was chosen to (1) separate the infusional toxicity of stem cells and CAR T cells, (2) take advantage of homeostatic cytokines driving lymphocyte recovery, and (3) separate toxicities associated with T-cell expansion from neutrophil engraftment syndrome after HSCT.
The attributes of specific T-cell subpopulations that enable them to sustain a functional immune response following adoptive transfer of in vitro propagated T cells has been the subject of intensive investigation. We demonstrated in a nonhuman primate model and human T-cell NOD/Scid interleukin (IL)-2RγCnull (NSG) mouse model that CD8+ effector T cells derived from macaque CD62L+CD95+ or CD62L+CD45RO+central memory T (TCM) cells, respectively, have the capacity to persist following adoptive transfer and repopulate functional memory niches.19,20 Consistently, Busch et al21 demonstrated the self-renewal capacity and multipotency of single TCM in serial transfer design, indicating the stemness of TCM. We therefore developed a clinical platform for purification, transduction, and expansion of CD19 CAR TCM for adoptive immunotherapy,22 with the goal of long-term CAR T–cell disease surveillance. We are optimizing this platform in a series of translational studies and here report on the first 2 phase 1 safety trials in which CD19 CAR TCM were administered 2 to 3 days following autologous HSCT in patients with relapsed or refractory NHL.
Methods
Clinical protocol design
The 2 trials described are phase 1 dose escalation protocols approved by the City of Hope Internal Review Board. Both the NHL1 and NHL2 trials followed the treatment schema depicted in Figure 1. Patients underwent leukapheresis for isolation of T cells for ex vivo selection and lentiviral transduction. Salvage chemotherapy was optional per standard practice as prescribed by the treating hematologist who selected agents best suited for individuals based on their response to prior therapy and organ toxicity considerations. Mobilization for autologous stem cell collection with cytoreductive chemotherapy (if no salvage given) and granulocyte colony-stimulating factor and/or plerixafor was administered per current City of Hope Standard Operating Policies, Procedures and Protocols. Research participants with evidence of progressive disease after salvage chemotherapy were not considered for the treatment phase of this study. HSCT conditioning regimen was not specified; however, all patients received bis-chloroethylnitrosourea, etoposide, Ara-C, and melphalan, and CD19 CAR T cells were infused 2 to 3 days after stem cell infusion.
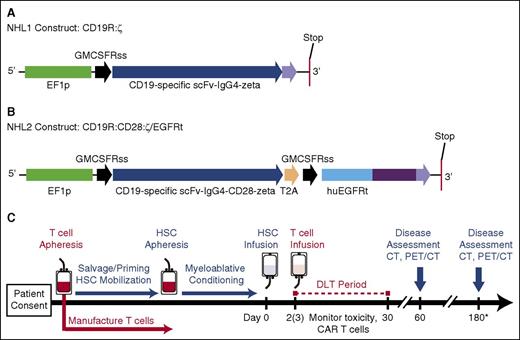
Constructs and treatment schema for clinical trials. (A) The CD19R:ζ DNA sequence (optimized by GeneArt) that contains the CAR sequence consisting of the VH and VL gene segments (scFv) of the CD19-specific FMC63 monoclonal antibody (mAb), IgG4 hinge-CH2-CH3, and signaling domains CD3ζ (CD19CAR) was cloned into a self-inactivating lentiviral vector pHIV7 that has an EF-1 promoter. (B) In addition to CD19 scFv, the CD19R:CD28:ζ/EGFRt+ epHIV7 lentiviral construct also contains (1) the cytoplasmic domain of the CD3ζ chain; (2) the CD28 costimulatory domain, (3) the self-cleaving T2A sequence; and (4) the truncated EGFR sequence as indicated. The huEGFRt was synthesized by PCR splice overlap extension to fuse in frame the human granulocyte-macrophage–colony-stimulating factor receptor's leader peptide to domains III and IV and the transmembrane spanning components of huEGFR (base pairs, 1000-2004). This fusion product was then cloned into the epHIV7 vector (in which the cytomegalovirus promoter of pHIV7 was replaced with an EF-1 promoter) along with the CD19CAR and T2A sequences, and the final construct was confirmed by sequence analysis. (C) Leukapheresis for T-cell manufacturing may be drawn before or after cycles of salvage chemotherapy. Research participants received T-cell infusions on either day +2 or +3 after HSCT. *Disease assessments continue every 6 months until 2 years after HSCT (6, 12, 18, and 24 months). PET, positron emission tomography.
Constructs and treatment schema for clinical trials. (A) The CD19R:ζ DNA sequence (optimized by GeneArt) that contains the CAR sequence consisting of the VH and VL gene segments (scFv) of the CD19-specific FMC63 monoclonal antibody (mAb), IgG4 hinge-CH2-CH3, and signaling domains CD3ζ (CD19CAR) was cloned into a self-inactivating lentiviral vector pHIV7 that has an EF-1 promoter. (B) In addition to CD19 scFv, the CD19R:CD28:ζ/EGFRt+ epHIV7 lentiviral construct also contains (1) the cytoplasmic domain of the CD3ζ chain; (2) the CD28 costimulatory domain, (3) the self-cleaving T2A sequence; and (4) the truncated EGFR sequence as indicated. The huEGFRt was synthesized by PCR splice overlap extension to fuse in frame the human granulocyte-macrophage–colony-stimulating factor receptor's leader peptide to domains III and IV and the transmembrane spanning components of huEGFR (base pairs, 1000-2004). This fusion product was then cloned into the epHIV7 vector (in which the cytomegalovirus promoter of pHIV7 was replaced with an EF-1 promoter) along with the CD19CAR and T2A sequences, and the final construct was confirmed by sequence analysis. (C) Leukapheresis for T-cell manufacturing may be drawn before or after cycles of salvage chemotherapy. Research participants received T-cell infusions on either day +2 or +3 after HSCT. *Disease assessments continue every 6 months until 2 years after HSCT (6, 12, 18, and 24 months). PET, positron emission tomography.
The NHL1 trial tested CD8-enriched TCM-derived cells transduced with a first-generation CD19 CAR that did not include a costimulatory domain (clinicaltrials.gov #NCT01318317, IND 14645). The NHL2 trial tested bulk TCM-derived cells, in which CD8 cells were not enriched, and the product contained both CD4 and CD8 T cells. These bulk TCM cells were then transduced with a second-generation CD19 CAR that included a CD28 costimulatory domain (clinicaltrials.gov #NCT01815749, IND 15490). The primary objectives for both trials were (1) to assess the safety and describe the full toxicity profile of CD19 CAR TCM-enriched cellular immunotherapy in conjunction with autologous myeloablative HSCT for patients with intermediate grade B-cell NHL and (2) to determine the maximum tolerated dose (MTD) based on dose-limiting toxicity (DLT). Both phase 1 studies used the toxicity equivalence range design, which defines the dose escalation and de-escalation rules for determining the MTD based on a target range of acceptable toxicity.23
Toxicity and disease assessment.
Toxicity was assessed using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE v4.03). Patients were followed for possible DLT during the 28 days following TCM cell infusion. DLT was defined as (1) any grade 3 or higher toxicity with an attribution of definitely or probably related to the infusion of the TCM cells (excepting expected infusion-related reactions and cytokine release syndrome (CRS) lasting <48 hours); (2) any toxicity requiring the use of steroids to ablate side effects attributable to the infusion of the TCM cells; (3) any lower grade toxicity that increases to a grade 3 or higher as a direct result of the TCM, (4) any grade 2 or greater autoimmune toxicity, and (5) failure of hematopoietic engraftment by day 21 after HSCT. Toxicities of any grade normally expected with an autologous stem cell transplant were not considered DLTs. Disease assessments were based on either computed tomography (CT) or PET/CT at baseline (pre-HSCT) and at 60 days, 6 months, 12 months, 18 months, and 24 months after HSCT as per standard of care.
CAR T–cell dose levels.
For the NHL1 trial, the originally proposed dose levels included major escalation steps at 50 × 106, 100 × 106, 500 × 106, and 1000 × 106 total T cells in a single infusion and possible half-step doses to be used in the event of DLT. After the first 3 patients, doses were based on CAR T cells rather than total T cells to more accurately review toxicities associated with a cell dose of the active product. For the NHL2 trial, all doses were based on CAR+ T cells, and the proposed dose escalation levels were 50 × 106, 200 × 106, and 800 × 106 CAR+ T cells in a single infusion.
Patient eligibility.
Eligible patients were ≥18 years old with a diagnosis of intermediate-grade B-cell NHL, ie, DLBCL, MCL, or transformed follicular lymphoma. Patients must have had a history of relapse after achieving first remission with primary therapy or failure to achieve remission with primary therapy and considered candidates for autologous HSCT. The NHL2 trial also allowed patients with high-risk disease in first remission as defined by poor prognosis histology (eg, MCL, double-hit DLBCL). Patients who had previously undergone either autologous or allogeneic HSCT were not eligible. All patients enrolled and treated on these trials gave written informed consent before participation; trials were conducted in accordance with the Declaration of Helsinki.
Generation of TCM-derived CD19 CAR T cells
For the NHL1 trial, TCMs were purified, transduced, formulated, and released as previously described.22 Detailed methods and differences between the protocols are described in supplemental Methods and supplemental Figure 1, available on the Blood Web site. For the NHL2 trial, several modifications were made to the published manufacturing procedures: (1) anti-CD4 microbeads were not used to deplete helper T cells, (2) anti-CD25 microbeads were added to remove T-regulatory cells, and (3) the median number of days of bead stimulation was shortened from 17 to 7 for NHL2. The lentiviral vectors encoding the first-generation CD19R:ζ construct or the second-generation CD19R:CD28:ζ/EGFRt construct are shown in Figure 1A-B. The cell products were released via serial quality control tests as described in supplemental Table 1.
Sample processing and storage for correlatives is described in supplemental Methods.
Results
Patient accrual and characteristics
NHL1 patients.
On NCT01318317, 13 participants consented, 1 went to hospice before leukapheresis, and 1 withdrew before leukapheresis. Eleven patients underwent leukaphereisis. Two patients did not qualify for HSCT, and 1 refused further treatment. Thus, 8 patients received HSCT and CD19 CAR TCM cell infusions; 7 (88%) had a diagnosis of DLBCL and 1 (13%) had a diagnosis of MCL. At baseline (prior to conditioning), 3 (38%) participants had a complete response (CR), 2 (25%) had a partial response (PR), 2 (25%) had stable disease (SD), and 1 (13%) had failed primary induction with stable disease after salvage therapy at transplant. Four of the 8 patients were women (50%), and 4 (50%) were ≥65 years of age. The mean age was 62 years (range, 50-75 years). The median number of prior chemotherapy regimens was 2 (range, 2-3). All patients received rituximab within 6 weeks prior to protocol treatment. Two of the 8 patients (25%) had received prior involved field radiation therapy. Table 124 describes individual patients treated at each dose, including baseline characteristics.
NHL2 patients.
On NCT01815749, 10 patients consented and underwent leukapheresis. One patient could not mobilize adequate stem cells for HSCT, and 1 patient was discovered to have a cytogenetic abnormality associated with myelodysplastic syndrome and was therefore ineligible for HSCT. Eight patients received HSCT and CD19 CAR T–cell infusion. Of these 8 treated patients, 4 (50%) had MCL and 4 (50%) had DLBCL. At baseline disease assessment, 6 (75%) participants had a CR and 2 (25%) had a PR. Three of the 8 were women (38%), and 2 (25%) were ≥65 years of age. The mean age was 58 years (range, 23-71 years). All patients had previously received rituximab at a median of 3.8 months (range, 1.8-15.4 months) prior to HSCT. The median number of prior chemotherapy regimens was 2 (range, 1-3). Table 224 describes individual patients treated at each dose, including baseline characteristics.
Dose escalation
Table 124 shows the number of patients treated at each CAR+ T-cell dose on the NHL1 trial. One patient was treated with 25 × 106 CAR+ T cells (50 × 106 total T cells), 4 patients were treated with ∼50 × 106 CAR+ T (dose level 1) cells, and 3 patients received 100 × 106 CAR+ T cells (dose level 2). Dose level 1 was overfilled to allow a patient that urgently needed treatment to enter the currently defined safe dose before the next cohort was open. No DLTs were observed at any dose. In lieu of escalating to the MTD on the NHL1 trial, we closed the trial to enrollment and opened the NHL2 trial, which tested a modified CAR vector including a CD28 costimulatory domain transduced into an enriched CD4+/CD8+ T-cell product. Table 224 shows the number of patients treated at each CAR+ T-cell dose on the NHL2 trial. Three patients completed the 50 × 106 CAR+ T-cell dose (dose level 1) with no DLTs, and the dose was escalated to 200 × 106 CAR+ T cells (dose level 2). Five patients were treated at dose level 2, which was overfilled to allow patients who already had manufactured T cells and qualified for protocol treatment to enter the current safe dose without waiting for the next cohort to open. Rather than escalating to MTD in the NHL2 trial, we elected to close the trial to enrollment after the 200 × 106 CAR+ T-cell dose and opened a new trial using a modified CAR vector construct.
TCM CD19 CAR T–cell manufacturing
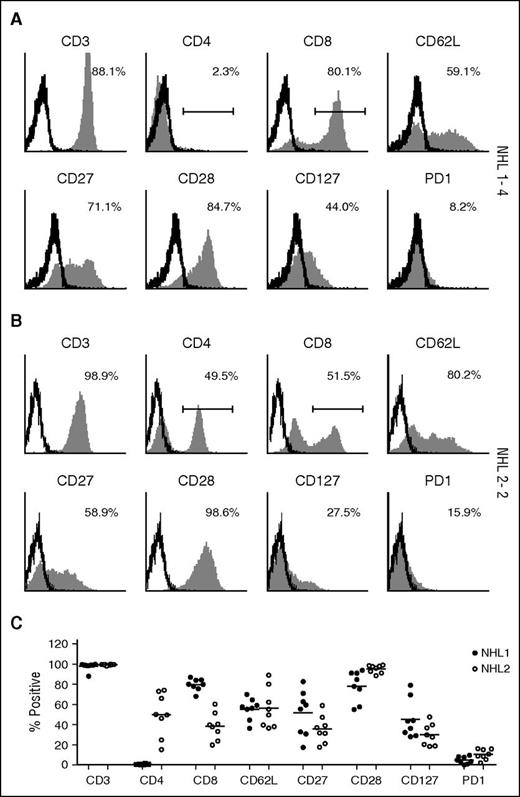
TCM CD19 CAR T–cell products were successfully generated in all patients that were leukapheresed, and all met quality control criteria and dose requirements. Manufactured products in both studies retained expression of central memory markers (CD62L, CD27, CD28, CD127), with NHL1 products including only CD8+ T cells and NHL2 including CD8+ and CD4+ T cells (Figure 2). We also modified the manufacturing process by shortening the bead stimulation time from a median of 17 days (range, 14-19 days) in the NHL1 trial to 7 days in the NHL2 trial, which decreased the ex vivo expansion period from a median of 28.5 days (range, 22-34 days) to a median of 24 days (range, 21-28 days) (supplemental Tables 2 and 3).
Surface phenotype of TCM-enriched CD19R+ cells. Freshly thawed T-cell products of (A) patient NHL1-4 and (B) patient NHL 2-2 were examined for the expression of CD3, CD4, CD8, CD62L, CD28, CD27, CD127, and PD-1 by flow cytometry. Percentages of positive cells (filled histograms) are calculated using the subtraction method on the basis of the gating of isotype-stained cells (open histograms), except CD4 and CD8 using indicated gating strategy. (C) Percentages of positive cells from all the patients are depicted. NHL1 patients are represented by filled circles and NHL2 patients by open circles. Means are presented for each surface phenotype.
Surface phenotype of TCM-enriched CD19R+ cells. Freshly thawed T-cell products of (A) patient NHL1-4 and (B) patient NHL 2-2 were examined for the expression of CD3, CD4, CD8, CD62L, CD28, CD27, CD127, and PD-1 by flow cytometry. Percentages of positive cells (filled histograms) are calculated using the subtraction method on the basis of the gating of isotype-stained cells (open histograms), except CD4 and CD8 using indicated gating strategy. (C) Percentages of positive cells from all the patients are depicted. NHL1 patients are represented by filled circles and NHL2 patients by open circles. Means are presented for each surface phenotype.
TCM CD19 CAR T–cell infusion-associated toxicity
As expected, all participants on NHL1 (n = 8) and NHL2 (n = 8) experienced Common Terminology Criteria for Adverse Events v 4.03 grade 4 hematologic toxicities, and 8 of 8 from NHL1 and 7 of 8 from NHL2 experienced grade 3 nonhematologic toxicities that were attributed at the probable or definite level to HSCT. There were no grade 5 toxicities (resulting in death). Infusion of the TCM CD19 CAR T cells was well tolerated, and no grade 2 or higher toxicities were attributed at the probable or definite level to the TCM CD19 CAR T–cell infusion. No patients required tocilizumab or steroid intervention as a result of CD19 CAR T cell–related toxicity.
CAR T cell in vivo expansion and persistence
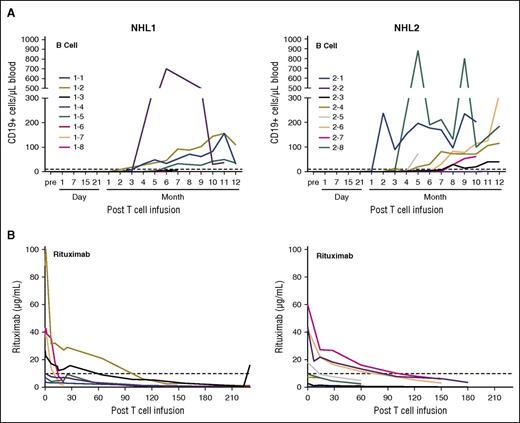
Patient peripheral blood samples were assayed by flow cytometry after infusion to detect antibody-stained CD19 CAR T cells. For the NHL1 trial samples, we used antibodies against the CD19scFv, and for NHL2 trial, we used cetuximab, which recognizes the truncated EGFR in the second-generation CAR construct (Figure 3). We used a quantitative polymerase chain reaction (qPCR) assay to detect woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) and determine lentiviral vector transgene copy number to enable quantitative tracking of CD19 CAR T cells in the blood. Both methods showed that CD19 CAR T cells reached peak frequencies at ∼2 weeks. The median peak expansion expressed in CAR copy number/mL of blood (based on WPRE) was 280 (range, 0-925; n = 7) in NHL1 and 692 (range, 267-27 790; n = 8) in NHL2 (Figure 4A-B). The mean persistence of quantifiable CD19 CAR was 18.25 days (standard deviation, 12.1; range, 0-28) for NHL1 and 20.5 days (standard deviation, 6.9; range, 7-27) for NHL2. CD19 CAR copy number/μg of genomic DNA (gDNA) from day 1 to 28 after infusion is plotted in Figure 4C-D. When expressed as area under the curve (AUC) in Figure 4E, CAR copy number/μg of DNA over 25 days was statistically significantly higher for the NHL2 trial than the NHL1 trial, using a Welch 2-sample 2-tailed t test: mean difference = 14.8 (95% confidence interval [CI], 7.4-22.3; P < .001). Peak expansion was also improved for NHL2 with a mean difference of 1.19 log10 copies/μg of DNA (95% CI, 0.54-1.83; t test, P = .002; Figure 4F), whereas persistence was not significantly different between the 2 trials (Figure 4G), with a mean difference of 2.25 days (95% CI, −8.56 to 13.1; t test, P > .5).
CD19 CAR T–cell persistence by flow cytometry. PBMCs from patient blood were analyzed by flow cytometry at different time points after adoptive transfer. (A) Live cells were identified by staining with 7-amino actinomycin D (7-AAD) and then gated on CD3 (either fluorescein isothiocyanate [FITC] or allophycocyanin [APC] labeled), with dump antibodies anti-CD14 (either phycoerythrin [PE] or FITC labeled), and anti-CD16 (either PE or FITC labeled). (B) Anti-CD19scFv CAR Alexa Fluor 647 antibody was used for detection of CD19-specific CAR+ T cells. Percentages of the highest CAR-positive cells at the indicated time points in all NHL1 patients are depicted. (C) Erbitux (cetuximab)-biotin/streptavidin-PE was used for detection of CD19 specific CAR+ T cells. Percentages of the highest CAR-positive cells at the indicated time points in all NHL2 patients are depicted. (D) Time course for a representative patient sample from NHL1 shows CAR T cells at baseline and various points after adoptive transfer. (E) Time course for a representative patient sample from NHL2 shows CAR T cells at baseline and various points after adoptive transfer.
CD19 CAR T–cell persistence by flow cytometry. PBMCs from patient blood were analyzed by flow cytometry at different time points after adoptive transfer. (A) Live cells were identified by staining with 7-amino actinomycin D (7-AAD) and then gated on CD3 (either fluorescein isothiocyanate [FITC] or allophycocyanin [APC] labeled), with dump antibodies anti-CD14 (either phycoerythrin [PE] or FITC labeled), and anti-CD16 (either PE or FITC labeled). (B) Anti-CD19scFv CAR Alexa Fluor 647 antibody was used for detection of CD19-specific CAR+ T cells. Percentages of the highest CAR-positive cells at the indicated time points in all NHL1 patients are depicted. (C) Erbitux (cetuximab)-biotin/streptavidin-PE was used for detection of CD19 specific CAR+ T cells. Percentages of the highest CAR-positive cells at the indicated time points in all NHL2 patients are depicted. (D) Time course for a representative patient sample from NHL1 shows CAR T cells at baseline and various points after adoptive transfer. (E) Time course for a representative patient sample from NHL2 shows CAR T cells at baseline and various points after adoptive transfer.
Comparison of CAR T–cell persistence between NHL1 and NHL2 T-cell therapy. gDNA was extracted from frozen aliquots of whole blood and tested for the WPRE copy number by TaqMan qPCR. Average copy numbers are presented if ≥2 of 3 replicates generated a cycle threshold (Ct) value. In the time points where only 1 of 3 was detectible, actual value (not average) was plotted. Starting on day 1, participants were measured for WPRE every 7 days (±3 days) during the first 28 days on study and then monthly thereafter. (A) WPRE copy numbers from 1 mL blood collected from day 1 up to day 158 after T-cell infusion in the NHL1 trial, except patient NHL1-3 (who only had PBMC sample), are plotted (whole blood, N = 7). After day 28, there was no detectable WPRE for any patient. (B) WPRE copy numbers from 1 mL blood collected from day 1 up to day 157 after T-cell infusion in the NHL2 trial are plotted (whole blood, N = 8). After day 27, there was no detectable WPRE for any patient. (C) WPRE copy numbers from blood collected from day 1 through day 28 after T-cell infusion in the NHL1 trial are plotted as a function of log10 copies/µg of gDNA (whole blood, N = 7; PBMCs, N = 1; UPN043). Gray area at bottom of graph is below the lower limit of quantification for the assay. (D) WPRE copy numbers from blood collected from day 1 through day 28 after T-cell infusion in the NHL2 trial are plotted as a function of log10 copies/µg of gDNA (whole blood, N = 8). Gray area at bottom of graph is below the lower limit of quantification for the assay. (E) The figure provides box and whisker plots of CAR copy number over time (AUC) by trial and dose level, with the individual patient AUCs shown in black. Box and whisker plots graphically present the median (heavy gray line), the mean (dashed line), low and upper quartiles (ends of the box), and minimum and maximum values. Outlier values are in red. The AUCs were calculated on the log10 WPRE values from day 1 through day 25 after T-cell infusion (see D-E). If a 25-day WPRE was not available, one was interpolated. Measurements that were considered below the limit of quantification were set to 4 copies/μg of gDNA. WPRE data were available from 8 participants (whole blood, N = 7; PBMCs, N = 1) from NHL1 and all 8 participants from NHL2 (whole blood, N = 8). Each participant had 5 to 8 measurements. The mean AUC for NHL2 was 40.2 copies × days/μg and the mean AUC for NHL1 was 25.4 copies × days/μg, giving a mean difference of 14.8 (95% CI: 7.4-22.3), with a P value for the Welch 2-sample 2-tail Student t test of <.001. (F) Mean peak expansion is the peak WPRE value measured in log10 CAR copies/µg of gDNA. Mean peak expansion for NHL1 was 1.6 copies/μg and for NHL2 was 2.79 copies/μg, giving a mean difference of 1.19 (95% CI: 0.54-1.83), yielding a P value of .002 using a t test. (G) Maximum persistence is the day associated with the last value above the lower limit of detection followed by 2 measurement that were below the limit of detection. Mean maximum persistence for NHL1 was 18.25 days and for NHL2 was 20.5 days, giving a mean difference of 2.25 (95% CI: −8.56 to 13.1), yielding a P value of >.5 using a t test.
Comparison of CAR T–cell persistence between NHL1 and NHL2 T-cell therapy. gDNA was extracted from frozen aliquots of whole blood and tested for the WPRE copy number by TaqMan qPCR. Average copy numbers are presented if ≥2 of 3 replicates generated a cycle threshold (Ct) value. In the time points where only 1 of 3 was detectible, actual value (not average) was plotted. Starting on day 1, participants were measured for WPRE every 7 days (±3 days) during the first 28 days on study and then monthly thereafter. (A) WPRE copy numbers from 1 mL blood collected from day 1 up to day 158 after T-cell infusion in the NHL1 trial, except patient NHL1-3 (who only had PBMC sample), are plotted (whole blood, N = 7). After day 28, there was no detectable WPRE for any patient. (B) WPRE copy numbers from 1 mL blood collected from day 1 up to day 157 after T-cell infusion in the NHL2 trial are plotted (whole blood, N = 8). After day 27, there was no detectable WPRE for any patient. (C) WPRE copy numbers from blood collected from day 1 through day 28 after T-cell infusion in the NHL1 trial are plotted as a function of log10 copies/µg of gDNA (whole blood, N = 7; PBMCs, N = 1; UPN043). Gray area at bottom of graph is below the lower limit of quantification for the assay. (D) WPRE copy numbers from blood collected from day 1 through day 28 after T-cell infusion in the NHL2 trial are plotted as a function of log10 copies/µg of gDNA (whole blood, N = 8). Gray area at bottom of graph is below the lower limit of quantification for the assay. (E) The figure provides box and whisker plots of CAR copy number over time (AUC) by trial and dose level, with the individual patient AUCs shown in black. Box and whisker plots graphically present the median (heavy gray line), the mean (dashed line), low and upper quartiles (ends of the box), and minimum and maximum values. Outlier values are in red. The AUCs were calculated on the log10 WPRE values from day 1 through day 25 after T-cell infusion (see D-E). If a 25-day WPRE was not available, one was interpolated. Measurements that were considered below the limit of quantification were set to 4 copies/μg of gDNA. WPRE data were available from 8 participants (whole blood, N = 7; PBMCs, N = 1) from NHL1 and all 8 participants from NHL2 (whole blood, N = 8). Each participant had 5 to 8 measurements. The mean AUC for NHL2 was 40.2 copies × days/μg and the mean AUC for NHL1 was 25.4 copies × days/μg, giving a mean difference of 14.8 (95% CI: 7.4-22.3), with a P value for the Welch 2-sample 2-tail Student t test of <.001. (F) Mean peak expansion is the peak WPRE value measured in log10 CAR copies/µg of gDNA. Mean peak expansion for NHL1 was 1.6 copies/μg and for NHL2 was 2.79 copies/μg, giving a mean difference of 1.19 (95% CI: 0.54-1.83), yielding a P value of .002 using a t test. (G) Maximum persistence is the day associated with the last value above the lower limit of detection followed by 2 measurement that were below the limit of detection. Mean maximum persistence for NHL1 was 18.25 days and for NHL2 was 20.5 days, giving a mean difference of 2.25 (95% CI: −8.56 to 13.1), yielding a P value of >.5 using a t test.
Clinical outcomes
We observed normal HSCT-associated neutrophil and platelet engraftment within 21 days in all 16 patients treated.
NHL1 trial.
Five of 8 (63%) participants on NHL1 achieved a best response of CR or continuing CR (3 patients in CR before HSCT); 1 patient achieved best response of PR, 1 continuing PR, and 1 patient progressed through protocol therapy. Four of 8 patients (50%), with a 95% binomial CI of 16% to 84% were progression free at both the 1- and 2-year evaluation. Median follow-up was 25.8 months (range, 24-37.3 months) for patients that had not yet progressed. There were 2 deaths, both from disease progression, 1 in a patient at the 50 × 106 CAR+ dose level and 1 at the 100 × 106 CAR+ dose level.
NHL2 trial.
All 8 NHL2 participants achieved a best response of CR or continuing CR (6 patients in CR before HSCT). Six of 8 (75%; 95% binomial CI, 35-97%) were progression free at the 1-year evaluation. Median follow-up for patients that had not yet progressed was 12.3 months (range, 12.2-14.1 months). There was 1 death from disease progression in a patient at the 200 × 106 CAR+ dose level.
Surrogates for activity: cytokine levels and B-cell reconstitution
Although we did not observe TCM CD19 CAR T–cell infusion-related clinical symptoms, we analyzed our patient samples for cytokine levels. Serum cytokine levels across dose cohorts for our patients were moderately increased, including IL-6 and interferon-γ (Figure 5), as well as IL-10, tumor necrosis factor α, granulocyte-macrophage–colony-stimulating factor, macrophage inflammatory protein-1 α, and IL-2Rα (supplemental Figure 2).
Cytokine profiles after HSCT and CD19 CAR T–cell infusion. Patient serum samples were analyzed with the Luminex IS100 bead array technology, and kits were purchased from Life Technologies (Invitrogen). The assays were performed by Clinical Immunobiology Correlative Studies laboratory (CICSL) at COH using commercially available 30-plex cytokine detection assays. Each data point represents the average of triplicate measurement. (A) Interferon-γ is plotted in pg/mL at weekly time points through day 28. (B) IL-6 is plotted in pg/mL at weekly time points through day 28.
Cytokine profiles after HSCT and CD19 CAR T–cell infusion. Patient serum samples were analyzed with the Luminex IS100 bead array technology, and kits were purchased from Life Technologies (Invitrogen). The assays were performed by Clinical Immunobiology Correlative Studies laboratory (CICSL) at COH using commercially available 30-plex cytokine detection assays. Each data point represents the average of triplicate measurement. (A) Interferon-γ is plotted in pg/mL at weekly time points through day 28. (B) IL-6 is plotted in pg/mL at weekly time points through day 28.
CD19 CAR T cells not only eliminate malignant CD19+ B cells but also normal B cells. Therefore, B-cell reconstitution could be a surrogate end point for effective T-cell therapy. B-cell reconstitution for both trials was observed starting at ∼3 months after CD19 CAR T–cell infusion. The median absolute number of CD19+ cells in patient peripheral blood at day 180 was 25.6/μL (range, 0-700/μL; N = 6) for the NHL1 trial and 14.7/μL (range, 0-190/μL; N = 7) for the NHL2 trial (Figure 6A).
B-cell reconstitution following HSCT and CD19 CAR T–cell infusion. PBMCs from patient blood were analyzed by flow cytometry at different time points after adoptive transfer. (A) Samples were analyzed by multiflow cytometry after staining with 7-AAD, CD3 VioBlue, CD19 PE, CD20 FITC, and CD10 APC for the analysis of B-cell reconstitution. Absolute CD19+ cells were calculated based on the percentage of CD19+ cells, white blood cell counts, and percentage of lymphocytes. (B) Rituximab levels in the serum of the patients were determined with a time-resolved fluorometry assay.
B-cell reconstitution following HSCT and CD19 CAR T–cell infusion. PBMCs from patient blood were analyzed by flow cytometry at different time points after adoptive transfer. (A) Samples were analyzed by multiflow cytometry after staining with 7-AAD, CD3 VioBlue, CD19 PE, CD20 FITC, and CD10 APC for the analysis of B-cell reconstitution. Absolute CD19+ cells were calculated based on the percentage of CD19+ cells, white blood cell counts, and percentage of lymphocytes. (B) Rituximab levels in the serum of the patients were determined with a time-resolved fluorometry assay.
B-cell aplasia can also be related to the presence of rituximab in the serum, a CD20 targeting monoclonal antibody that is routinely administered to patients with NHL. Because all patients received rituximab treatment at some point prior to HSCT and CD19 CAR T–cell infusion, serum levels of rituximab were analyzed at different time points after HSCT to better understand the kinetics of B-cell reconstitution. The half-life of rituximab can vary depending on patients’ CD19-positive cell counts, tumor burden, and cycles of the treatment,25,26 In our trials, rituximab was detectable in the serum of some patients above the redosing level of 10 μg/mL for up to ∼3 months after CD19 CAR T–cell infusion (Figure 6B).
Discussion
Our team has developed CD19 CAR T–cell therapy for use in conjunction with autologous HSCT with the aim of reducing the relapse rate after transplantation. We report here on the NHL1 and NHL2 clinical trials testing successive modifications of our CD19 CAR vector design and manufacturing platform. Data from these phase 1 clinical trials support the safety and feasibility of administering TCM-derived CD19 CAR T cells for NHL patients following HSCT.
In both trials, hematopoietic stem cell engraftment was unperturbed, and the only grade 3 or 4 toxicities were those expected from myeloablative HSCT. Adoptive cellular immunotherapy, particularly with genetically modified T cells, has been associated with several potential safety concerns such as CRS and neurologic changes, both of which have been associated with marked increase in serum cytokine levels.8,11 In our studies, we noted no grade 2 or greater toxicities probably or definitely attributable to T cells and saw only mildly increased cytokine levels. Although NHL2 T cells expanded ∼2 logs (Figure 4D), peak levels (0.27-28 CAR copies/µL blood) were still below those seen by Kochenderfer et al16 (9-777 CAR-positive T cells/µL blood) in NHL patients with active disease. This may be related in part to the low cell doses tested and the low CD19 antigen drive in our HSCT trials. The Memorial Sloan Kettering Cancer Center group has also applied CAR T-cell therapy after HSCT in patients with relapsed/refractory aggressive B-cell NHL and recently presented interim data.27 At the 5 × 106/kg T-cell dose, there were 7 patients treated, with 1 experiencing DLT of grade III to IV cytopenias and death from infection . One patient was treated with 107 CAR T cells/kg and experienced a DLT of grade 4 severe CRS. Four patients total experienced CRS that was manageable by tocilizumab ± steroids. It is noteworthy that the CAR T–cell dose used on the Memorial Sloan Kettering Cancer Center trial of ≥350 × 106 CAR T cells (for a 70-kg patient) exceeds our highest tested dose of 200 × 106 CAR T cells.
The NHL1 trial used CD8+ TCM for CAR transduction; however, the presence of CD4+ T cells was later shown to enhance the functionality of CD8+ T cells,28,29 so we modified the manufacturing platform for the NHL2 trial by removing the CD4 depletion step. Another difference between the 2 trials was the addition of the CD28 costimulatory domain in the NHL2 CD19 CAR. Studies have shown that costimulatory molecules are critical for CAR T–cell expansion and persistence following adoptive therapy.30-32 Additionally, for NHL2 manufacturing, the CD25 depletion step was added, and ex vivo culture times were slightly shorter (24 vs 28.5 days). Comparing NHL1 and NHL2 in the same disease settings, we saw a statistically significant enhancement of expansion of CD19 CAR T cells in NHL2 patients.
CD19 CAR T–cell activity is difficult to assess by disease response, because 9 of 16 patients were in CR at start of study, and HSCT can also produce CRs. B-cell recovery was examined as a potential surrogate for in vivo effector function of transferred autologous CD19 CAR T cells. In most patients on both trials, B-cell reconstitution was observed around 3 months after CD19 CAR TCM infusion; however, serum rituximab levels in many patients remained above the redosing level (10 µg/mL) for 3 months. Thus, timing of normal B-cell reconstitution may reflect prior rituximab treatment. Long-term follow-up of the patients and evaluation of PFS outcomes will be more informative with regard to CD19 CAR TCM activity. At this point, with short duration of follow-up, we see a PFS of 50% for NHL1 at 1 and 2 years and 75% for NHL2 at 1 year after HSCT; given the small sample size, this difference is statistically indistinguishable.
These are the first reported clinical studies using TCM-derived CD19 CAR T cells for the treatment of B-cell malignancy in the setting of autologous HSCT, and we include 1 year of follow-up data on all patients. On the basis of preclinical data,19,20 we hypothesized that enriching the TCM population would provide long-term persistence and immunologic memory. TCM products from both of our trials showed peak levels in the blood at ∼14 days, trailing off after ∼28 days. Thus, a T-cell product derived from central memory enrichment as described in these studies does not persist longer than what is observed in trials with conventional bulk T cells transduced with CARs bearing CD28 costimulatory domains.10,16,27,33 The costimulatory domain seems to have a dominant effect on persistence, because CD28 CAR T cells typically persist for ∼1 month,9 whereas CARs bearing 4-1BB costimulatory domains have been detected in patient blood for up to 4 years.34 It is also possible that the potential benefits of the TCM selection were offset by longer ex vivo expansion times (24 days) compared with peripheral blood mononuclear cell (PBMC)-derived CAR T cells from lymphoma patients (10 days).16
In contrast to the high levels of in vivo expansion of CD19 CAR T cells seen in studies treating high disease burden,8 we detected relatively low levels of CD19 CAR TCM expansion in the circulation using both cellular and molecular techniques. This is possibly related to the lack of antigen drive (ie, disease burden or normal B cells) at the time of CD19 CAR TCM infusion following myeloablative conditioning. Modifying the manufacturing platform to include CD4 cells, shortening culture time, and adding a CD28 costimulatory domain to the CAR resulted in modest augmentation of CD19 CAR TCM peak expansion and CAR levels over time as assessed by AUC.
It is likely that factors other than cell origin play a role in the persistence of transferred cells and their therapeutic efficacy. We are currently accruing patients to a phase 1 clinical trial (#NCT02051257) of CD19 CAR T cells after HSCT using a hinge-optimized CAR mutated in the CH2 region of the immunoglobulin (Ig)G4-Fc spacer to reduce Fc binding, which improved persistence and antilymphoma efficacy in NSG mice.35 Additional studies in the setting of HSCT will explore changing the starting T-cell population, further shortening manufacturing time, and comparing 4-1BB as a costimulatory molecule.
On the basis of findings in these sequential phase 1 trials, we conclude that TCM-derived CD19CAR T–cell therapy, using the methods herein described, is feasible and safe, and does not increase toxicity or delay hematopoietic engraftment of high-risk NHL patients undergoing HSCT. Neither trial escalated to MTD, as we opted to implement CAR and manufacturing improvements, offering patients the most promising protocols available at the time. We continue long-term follow-up of these patients to assess impact on HSCT outcomes. Analysis of patient samples from these trials along with concurrent preclinical experiments has allowed us to further refine the CAR vectors and manufacturing processes. The next trial in this series of clinical studies is underway, with the goal of extending the duration and potency of antitumor immunity in the setting of immune reconstitution following autologous transplantation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Tim Nesvig Lymphoma Research Fund and the Skirball Foundation and two grants from the National Cancer Institute of the National Institutes of Health: the City of Hope Lymphoma Specialized Programs of Research Excellence (SPORE) grant P50 CA107399 and the City of Hope Cancer Center Support grant P30 CA33572.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: X.W., J.R.W., A.N., W.-C.C., M.C.J., S.R.R., C.E.B., and S.J.F. assisted in CAR and product design; X.W., J.R.W., A.N., M.C.J., C.E.B., and S.J.F. assisted in CAR and T-cell manufacturing; L.L.P., J.R.W., M.S.B., M.C.J., and S.J.F. designed the clinical trial; L.L.P., J.R.W., M.R.M., A.P.N., and S.J.F. executed the clinical trial; L.J.N.C. provided the anti-CD19scFv antibody; X.W., C.W.W., R.Z.U., B.C., C.E.B., and S.J.F. contributed correlative studies; L.L.P., A.N., M.R.M., B.C., J.X., and M.S.B. collected data; X.W., L.L.P., J.R.W., A.N., M.S.B., C.W.W., R.Z.U., S.K.K., T.S., L.E.B., S.H.T., N.G., C.E.B., and S.J.F. provided data analysis and interpretation; X.W., L.L.P., J.R.W., M.S.B., J.X., S.H.T., C.E.B., and S.J.F. prepared the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: M.C.J. and S.R.R. have ownership interests in Juno Therapeutics. L.J.N.C. is currently the Chief Executive officer of Ziopharm Oncology but was employed at M.D. Anderson at the time these studies were undertaken. He also has the following disclosures: intellectual property/patent holder (Sangamo BioSciences), ownership interest in Targazyme, Intrexon, Ziopharm, and Immatics. S.J.F. and C.E.B. have license agreements with and receive research support from Mustang Therapeutics. All other authors declare no competing financial interests.
Correspondence: Stephen J. Forman, 1500 E. Duarte Rd, Duarte, CA 91010; e-mail: sforman@coh.org.
References
Author notes
X.W., L.L.P., and J.R.W. contributed equally to this work.
C.E.B., M.C.J., and S.J.F. contributed equally to this work.

![Figure 3. CD19 CAR T–cell persistence by flow cytometry. PBMCs from patient blood were analyzed by flow cytometry at different time points after adoptive transfer. (A) Live cells were identified by staining with 7-amino actinomycin D (7-AAD) and then gated on CD3 (either fluorescein isothiocyanate [FITC] or allophycocyanin [APC] labeled), with dump antibodies anti-CD14 (either phycoerythrin [PE] or FITC labeled), and anti-CD16 (either PE or FITC labeled). (B) Anti-CD19scFv CAR Alexa Fluor 647 antibody was used for detection of CD19-specific CAR+ T cells. Percentages of the highest CAR-positive cells at the indicated time points in all NHL1 patients are depicted. (C) Erbitux (cetuximab)-biotin/streptavidin-PE was used for detection of CD19 specific CAR+ T cells. Percentages of the highest CAR-positive cells at the indicated time points in all NHL2 patients are depicted. (D) Time course for a representative patient sample from NHL1 shows CAR T cells at baseline and various points after adoptive transfer. (E) Time course for a representative patient sample from NHL2 shows CAR T cells at baseline and various points after adoptive transfer.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/24/10.1182_blood-2015-12-686725/4/m_2980f3.jpeg?Expires=1765934467&Signature=VunFcrVqMQJJ6z8BvlyTUyKIT2jM160zeGug7VHgri01zjc4gGaoiaScYoS159odkTZINXtM2uLN4OFtKc~L-5DpIGVG~9tBhT~V0ZQHYD0C6qnpnIbtihQrjsYPYigOJl~10cUaUqFDvLewJplt04G9vXQ87qcGHS6JclOSJyGnKDL0gFV8phP5y232DneOcHqi-aVQWApfFD2~kwdhanaiiGgBC2qPpF450SF~YbplfiP34gzK1JQaOk1LuuVJSjOdwkHziLSWhPoJILl-1vUTPz79bo1veTqBcHnmnKcftFKt0xs7dyjR0GK1BjTBCz6kcPv83VdHI~TmWb8ZGQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)