Key Points
HCK transcription and activation is triggered by mutated MYD88, and is an important determinant of pro-survival signaling.
HCK is also a target of ibrutinib, and inhibition of its kinase activity triggers apoptosis in mutated MYD88 cells.
Abstract
Activating mutations in MYD88 are present in ∼95% of patients with Waldenström macroglobulinemia (WM), as well as other B-cell malignancies including activated B-cell (ABC) diffuse large B-cell lymphoma (DLBCL). In WM, mutated MYD88 triggers activation of Bruton tyrosine kinase (BTK). Ibrutinib, a pleiotropic kinase inhibitor that targets BTK, is highly active in patients with mutated MYD88. We observed that mutated MYD88 WM and ABC DLBCL cell lines, as well as primary WM cells show enhanced hematopoietic cell kinase (HCK) transcription and activation, and that HCK is activated by interleukin 6 (IL-6). Over-expression of mutated MYD88 triggers HCK and IL-6 transcription, whereas knockdown of HCK reduced survival and attenuated BTK, phosphoinositide 3-kinase/AKT, and mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in mutated MYD88 WM and/or ABC DLBCL cells. Ibrutinib and the more potent HCK inhibitor A419259, blocked HCK activation and induced apoptosis in mutated MYD88 WM and ABC DLBCL cells. Docking and pull-down studies confirmed that HCK was a target of ibrutinib. Ibrutinib and A419259 also blocked adenosine triphosphate binding to HCK, whereas transduction of mutated MYD88 expressing WM cells with a mutated HCK gatekeeper greatly increased the half maximal effective concentration for ibrutinib and A419259. The findings support that HCK expression and activation is triggered by mutated MYD88, supports the growth and survival of mutated MYD88 WM and ABC DLBCL cells, and is a direct target of ibrutinib. HCK represents a novel target for therapeutic development in MYD88-mutated WM and ABC DLBCL, and possibly other diseases driven by mutated MYD88.
Introduction
Next-generation sequencing has revealed activating MYD88 mutations in several B-cell malignancies. Particularly striking has been the expression of MYD88 mutations in Waldenström macroglobulinemia (WM), wherein 95% to 97% of patients express MYD88L265P, and more rarely non-L265P MYD88 mutations.1-4 Up to 30% of patients with activated B-cell (ABC) subtype of diffuse large B-cell lymphoma (DLBCL) also express activating MYD88 mutations, including MYD88L265P.5,6 Mutations in MYD88 promote Myddosome self-assembly and can trigger NF-κB signaling in the absence of toll-like receptor or interleukin 1 receptor (IL-1R) signaling through IL-1 receptor associated kinases (IRAK4/IRAK1) or Bruton tyrosine kinase (BTK).5,7
Ibrutinib is a pleiotropic kinase inhibitor that is known to target BTK and is highly active in previously treated WM patients, producing an overall response rate of 91%.8 In WM patients, both major and overall responses to ibrutinib are higher in patients with MYD88 mutations.4 CXCR4 WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) mutations that are present in up to 40% of WM patients can also impact ibrutinib response.4 Ibrutinib also shows activity in previously treated patients with ABC-DLBCL, particularly among patients with the B-cell receptor (BCR) pathway and MYD88 mutations.9 Ibrutinib is also active in other B-cell malignancies including chronic lymphocytic leukemia and mantle cell lymphoma. Suppression of tonic BCR activity mediated by BTK has been implicated as the mechanism underlying ibrutinib activity in non-WM B-cell diseases.
In addition to BTK, ibrutinib can suppress the activity of several other kinases including members of the SRC family.10 Hematopoietic cell kinase (HCK) is a member of the SRC family of protein tyrosine kinases, and one of the most aberrantly upregulated genes in WM cells.11 In myeloma cells, HCK is activated by IL-6 through the IL-6 co-receptor IL-6ST (GP130).12,13 IL-6 is also a potent growth and survival factor in WM, though the functional significance of HCK in WM pathogenesis remains unclear.14 We therefore investigated the role of HCK in WM, and impact of MYD88 mutations and ibrutinib on the transcriptional regulation and activation of this SRC family member.
Materials and methods
Primary cells and cell lines
Primary WM cells (CD19+) were isolated from bone marrow (BM) aspirates, and peripheral blood mononuclear cells were collected from healthy donors (HDs) after informed written consent was obtained. Primary WM lymphoplasmacytic cells (LPCs) and cell lines were genotyped for MYD88 and CXCR4 mutations as previously described.2,4 MYD88L265P BCWM.1 and MWCL-1 WM cells; TMD-8, HBL-1, and OCI-Ly3 and MYD88S222R SU-DHL2 ABC DLBCL cells; and MYD88 wild-type (WT) (MYD88WT) OCI-Ly7 and OCI-Ly19 germinal center B-cell (GCB) DLBCL cells; Ramos Burkitt cells; and RPMI-8226 and MM.1S myeloma cells were used.
Lentiviral transduction experiments
MYD88WT or MYD88L265P proteins were over-expressed in BCWM.1 and MWCL-1 cells following lentiviral transduction as previously described.7 The over-expression of proteins coding for WT HCK (HCKWT) or HCK harboring a mutated gatekeeper residue (HCKT333M) at amino acid position 333 (338 based on c-SRC numbering)15 was accomplished by lentiviral transduction of HCKWT or HCKT333M (r. 1235C>T in NM_002110.3) sequences in a pLVX-EF1α-IRES-Puro Vector (Clontech Laboratories, Mountain View, CA). Knockdown of HCK or IL-6ST (GP130) was performed using an inducible lentiviral short hairpin RNA (shRNA) expression vector pLKO-Tet-On containing a tetracycline-regulated expression cassette (Addgene, Cambridge, MA). Following lentiviral transduction of BCWM.1 and MWCL-1 cells, stable cell lines were selected with 0.5 to ∼1.0 μg/mL puromycin in a tetracycline-free medium. For the induced knockdown of HCK or IL-6ST, tetracycline (0.8 μg/mL) was added to culture media. For HCK knockdown, lentiviral vectors were designed to target 5′-GCTGTGATTTGGAAGGGAA-3′ (HCK shRNA1) and 5′-GGATAGCGAGACCACTAAA-3′ (HCK shRNA2). IL-6ST knockdown targeted 5′-GGAGCAATATACTATCATA-3′ (IL-6ST shRNA1) and 5′-GGAACTGTCTAGTATCTTA-3′ (IL-6ST shRNA2). A scrambled shRNA vector was used for control purposes.
Cell viability assessments
Apoptosis analysis was performed using Annexin V/propidium iodide (PI) staining with the Apoptosis Detection Kit I (BD Pharmingen, San Jose, CA). Cells (1 × 106/well) were treated in 24-well plates for 18 hours with inhibitors or corresponding controls. A minimum of 10 000 events were acquired using a BD FACSCanto II flow cytometer, and analyzed with BD FACS DIVA software. For WM patient cells, BM mononuclear cells (2 × 106/well) were treated with inhibitors, and CD19–APC-cy7 antibody (BD Pharmingen) was used with Annexin V antibody to analyze WM cell apoptosis. AlamarBlue cell viability assay (Life Technologies, Carlsbad, CA) was used to assess cell death following inducible HCK knockdown. For these experiments, transduced cells (1 × 105/mL) were cultured with tetracycline, and aliquoted every other day on days 1 to 11. AlamarBlue solution (1/10 total volume) was added to cells and incubated for 2 hours. Aliquoted plates were read in a SpectraMax M3 Plate Reader (Molecular Devices, Sunnyvale, CA). Relative cell survival was calculated as a percentage of fluorescence relative to scrambled control. The CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison WI) was used to assess the dose-response of inhibitors. Cells were seeded into 384-well plates with the EL406 Combination Washer Dispenser (BioTek Instruments, Inc.) and inhibitors injected into the cells culture media with the JANUS Automated Workstation (PerkinElmer Inc., Waltham, MA). Cells were treated with serial diluted inhibitors (20 to ∼0.0006 μM) for 72 hours at 37°C. Luminescent measurement was performed using the 2104 EnVision Multilabel Reader (PerkinElmer Inc.).
Reverse transcription polymerase chain reaction (RT-PCR) and quantitative-PCR
Total RNA were isolated using AllPrep DNA/RNA Mini Kit (QIAGEN), and cDNA synthesized by SuperScript III First-Strand Synthesis SuperMix (Life Technologies). Quantitative detection of messenger RNA levels for HCK, IL-6 and IL-6R was performed using TaqMan Gene Expression Assays with TaqMan Gene Expression Master Mix per manufacturer’s instructions using the ABI Prism 7500 Sequence Detection System (Applied Biosystems). HCK transcription was also assessed two hours following incubation with either IL-6 (1-10 ng/mL) or IL-6 blocking antibody (1-10 μg/mL) (both from R&D Systems, Minneapolis, MN) in BCWM.1 and MWCL-1 cells by quantitative RT-PCR (qRT-PCR).
Phosflow analysis
Phosflow analysis was performed to delineate HCK phosphorylation. Cells were fixed with BD Phosflow Fix Buffer I at 37°C for 10 minutes. Cells were then centrifuged (300g for 5 minutes) and washed twice with Phosflow Perm/Wash Buffer I (BD Pharmingen). Cells were then stained with HCK (pTyr411) antibody (Abcam, Cambridge, MA) alone (for cell lines) or with anti-CD20–APC-Cy7 (BD Pharmingen) for primary WM cells. Following staining, cells were incubated in the dark for 30 minutes at room temperature, then washed thrice with BD Phosflow Perm/Wash Buffer I, followed by anti-rabbit immunoglobulin G DyLight-649 secondary antibody (Abcam), and incubated for an additional 20 minutes. Cells were then washed twice with BD Phosflow Perm/Wash Buffer I and flow analysis was performed using a BD FACSCanto II Flow Cytometer.
Immunoblotting
Immunoblotting was performed following gene over-expression, knockdown, or kinase pull-down with biotinylated probes using antibodies for HCK, AKT-pT308, AKT, extracellular signal-regulated kinase 1/2 (ERK1/2)-pT202/pY204, ERK1/2, PLCγ1-pY783, PLCγ2-pY1217, PLCγ2, BTK-pY223, BTK, IRAK4-pT345/S346, IRAK4, SRC, LYN (Cell Signaling Technologies, Danvers, MA), PI3K-p85β-pY464 (LifeSpan Biosciences, Seattle, WA), and PI3K-p85β, PLCγ1, IL-6ST (Santa Cruz Biotechnology, Dallas, TX) in primary WM cells, and cell lines. Staining with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies was used for determination of protein loading (Santa Cruz Biotechnology).
Ibrutinib probe assay and kinase active-site inhibition assay
For ibrutinib probe assay, BCWM.1, MWCL-1, or TMD-8 cells (2 × 107) were treated with ibrutinib-biotin or CC-292–biotin (2 μM) for 1 hour. Cells were then washed with phosphate-buffered saline twice, and lysed with co-IP buffer (Invitrogen, Grand Island, NY). Protein (2 mg) from lysed cells was then incubated with 50 μL of Pierce Streptavidin Magnetic Beads (Thermo Fisher Scientific, Cambridge, MA) at 4°C for 1 hour, then washed with Tris-buffered saline with Tween 20 (0.1%) thrice, and proteins eluted with sodium dodecyl sulfate–polyacrylamide gel electrophoresis sample buffer. For kinase active-site inhibition assay, BCWM.1 cells (2 × 107) were pre-treated with dimethyl sulfoxide (DMSO), ibrutinib, CC-292, or A419259 (MedChem Express, Monmouth Junction, NJ) at various concentrations for 1 hour. Cells then were lysed and kinases were pulled down with adenosine triphosphate (ATP)-biotin using the Pierce Kinase Enrichment Kit (Thermo Fisher Scientific) per the manufacturer’s instructions. Kinases were eluted with sodium dodecyl sulfate–polyacrylamide gel electrophoresis sample buffer and detected by western blot.
Docking study
Statistical analysis
The statistical significance of differences was analyzed using one-way analysis of variance with Tukey’s multiple comparisons test by Prism software. Differences were considered significant when P < .05.
Results
HCK transcription is driven by mutated MYD88
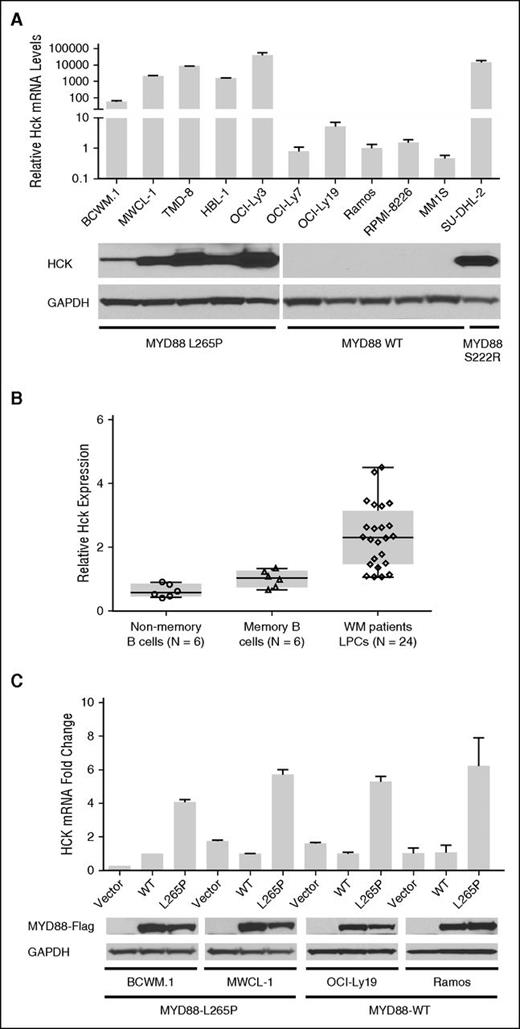
To clarify if HCK expression was aberrant in MYD88-mutated cells, we first assessed HCK transcription in MYD88-mutated WM and DLBCL cell lines by TaqMan Gene Expression Assay. The results showed that HCK was markedly transcribed in MYD88L265P expressing WM (BCWM.1 and MWCL-1) and DLBCL (TMD-8, HBL-1, and OCI-Ly3) cells, but absent or at very low levels in MYD88WT (OCI-Ly7, OCI-Ly19, Ramos, MM1.S, and RPMI-8226) cells by qRT-PCR (Figure 1A). Expression of the HCK transcript was also enhanced in SU-DHL2 cells that carry the MYD88S222R activating mutation. Western blot analysis confirmed enhanced HCK protein expression in MYD88-mutated cell lines (Figure 1A). We next investigated the messenger RNA levels of HCK in MYD88L265P genotyped CD19-sorted primary WM cells using a TaqMan Gene Expression Assay. We compared HCK expression levels to both sorted HD-derived nonmemory (CD19+CD27−) and memory (CD19+CD27+) B cells, given that the later represents the B-cell population from where most cases of WM are likely derived.19,20 The HCK transcript was elevated in MYD88L265P WM cells vs either HD nonmemory or memory B cells (Figure 1B). CXCR4 mutation status did not impact HCK transcription in primary WM cells (P = .90 for CXCR4 WT vs WHIM-mutated patients). To clarify if mutated MYD88 was responsible for enhanced HCK expression, we over-expressed MYD88L265P or MYD88WT protein in MYD88L265P BCWM.1 and MWCL-1 cells, and MYD88WT OCI-Ly9 and Ramos cells, and assessed for differences in HCK transcription. By western blot analysis, similar levels of exogenous MYD88 protein were detectable in MYD88L265P or MYD88WT transduced cells (Figure 1C). The results of these studies showed significantly higher levels of HCK transcript in all 4 cell lines following over-expression of MYD88L265P vs MYD88WT protein (Figure 1C).
HCK transcription is driven by mutated MYD88. (A) HCK transcript levels in MYD88-mutated WM (BCWM.1 and MWCL-1) and ABC DLBCL (TMD-8, HBL-1, OCI-Ly3, and SU-DHL2) cells, and MYD88 WT (OCI-Ly7 and OCI-Ly19 GCB DLBCL, Ramos Burkitt, MM1.S, and RPMI-8226 myeloma) cells by qRT-PCR. (B) HCK transcription using TaqMan Gene Expression Assay in primary LPCs (CD19+) from MYD88 L265P expressing WM patients, and HD-derived nonmemory (CD19+CD27−) and memory (CD19+CD27+) B cells. P < .01 for comparison of WM LPC vs either HD non-memory or memory B-cells. The number of healthy donors that were evaluated for nonmemory (open circles) and memory (open triangles) B cells, as well as the number of WM patients that were evaluated for LPC (open diamonds) are shown. (C) Fold change in HCK transcription following lentiviral transduction with vector control, Flag-tagged MYD88 L265P or MYD88 WT protein in cell lines expressing mutated or WT MYD88. At the bottom of (C), total Flag-tagged MYD88 L265P or WT protein levels are shown for all transduced cell lines to demonstrate relative translation efficiency for MYD88 L265P and WT vectors, as well as GAPDH protein levels as protein loading controls.
HCK transcription is driven by mutated MYD88. (A) HCK transcript levels in MYD88-mutated WM (BCWM.1 and MWCL-1) and ABC DLBCL (TMD-8, HBL-1, OCI-Ly3, and SU-DHL2) cells, and MYD88 WT (OCI-Ly7 and OCI-Ly19 GCB DLBCL, Ramos Burkitt, MM1.S, and RPMI-8226 myeloma) cells by qRT-PCR. (B) HCK transcription using TaqMan Gene Expression Assay in primary LPCs (CD19+) from MYD88 L265P expressing WM patients, and HD-derived nonmemory (CD19+CD27−) and memory (CD19+CD27+) B cells. P < .01 for comparison of WM LPC vs either HD non-memory or memory B-cells. The number of healthy donors that were evaluated for nonmemory (open circles) and memory (open triangles) B cells, as well as the number of WM patients that were evaluated for LPC (open diamonds) are shown. (C) Fold change in HCK transcription following lentiviral transduction with vector control, Flag-tagged MYD88 L265P or MYD88 WT protein in cell lines expressing mutated or WT MYD88. At the bottom of (C), total Flag-tagged MYD88 L265P or WT protein levels are shown for all transduced cell lines to demonstrate relative translation efficiency for MYD88 L265P and WT vectors, as well as GAPDH protein levels as protein loading controls.
IL-6 but not IL-6R transcription is induced by mutated MYD88
Because previous work showed that HCK was activated by IL-6 via IL-6R/IL-6ST, we investigated the regulatory role of mutated MYD88 on IL-6 and IL-6R expression.12,13 By qRT-PCR, IL-6 transcription was markedly higher in MYD88L265P expressing WM (BCWM.1 and MWCL-1) and DLBCL (TMD-8, HBL-1, and OCI-Ly3), as well as MYD88S222R expressing SU-DHL2 cells vs MYD88WT (OCI-Ly7, OCI-Ly19, Ramos, MM1.S, and RPMI-8226) cells (Figure 2A). Similarly, IL-6R transcription was increased in MYD88-mutated cell lines. Among MYD88WT malignant B cells, IL-6R transcription was low or absent, although it was highly expressed in MYD88WT malignant plasma cells (Figure 2B). By TaqMan Gene Expression Assay, higher IL-6 (Figure 2C) and IL-6R (Figure 2D) transcription were found in MYD88L265P WM samples vs HD non-memory (CD19+CD27−) and memory (CD19+CD27+) B cells. Given these findings, we next sought to clarify if MYD88L265P was a driver for IL-6 and IL-6R transcription. Over-expression of the MYD88L265P protein induced marked IL-6 (Figure 2E), but not IL-6R (Figure 2F) transcription in MYD88L265P (BCWM.1 and MWCL-1) and MYD88WT (OCI-Ly9 and Ramos) cells. Conversely, over-expression of MYD88WT protein had little or no impact on IL-6 or IL-6R transcription.
IL-6 but not IL-6R transcription is induced by mutated MYD88. Expression of IL-6 (A) and IL-6R (B) transcripts using TaqMan Gene Expression Assays in MYD88-mutated and MYD88 WT cell lines. The number of healthy donors that were evaluated for nonmemory (open circles) and memory (open triangles) B cells, as well as the number of WM patients that were evaluated for LPC (open diamonds) are shown. IL-6 (C) and IL-6R (D) transcript levels were also determined by TaqMan Gene Expression Assays in primary LPCs (CD19+) from MYD88 L265P expressing WM patients, and HD-derived nonmemory (CD19+CD27−) and memory (CD19+CD27+) B cells. P < .01 for comparison of IL-6 or IL-6R transcript levels in WM LPC vs either HD nonmemory or memory B cells. IL-6 (E) and IL-6R (F) transcription following over-expression of MYD88 L265P or WT protein in cell lines expressing mutated or WT MYD88. Total Flag-tagged MYD88 L265P or WT protein, as well as GAPDH protein levels determined by immunoblotting are shown for all transduced cell lines in Figure 1C.
IL-6 but not IL-6R transcription is induced by mutated MYD88. Expression of IL-6 (A) and IL-6R (B) transcripts using TaqMan Gene Expression Assays in MYD88-mutated and MYD88 WT cell lines. The number of healthy donors that were evaluated for nonmemory (open circles) and memory (open triangles) B cells, as well as the number of WM patients that were evaluated for LPC (open diamonds) are shown. IL-6 (C) and IL-6R (D) transcript levels were also determined by TaqMan Gene Expression Assays in primary LPCs (CD19+) from MYD88 L265P expressing WM patients, and HD-derived nonmemory (CD19+CD27−) and memory (CD19+CD27+) B cells. P < .01 for comparison of IL-6 or IL-6R transcript levels in WM LPC vs either HD nonmemory or memory B cells. IL-6 (E) and IL-6R (F) transcription following over-expression of MYD88 L265P or WT protein in cell lines expressing mutated or WT MYD88. Total Flag-tagged MYD88 L265P or WT protein, as well as GAPDH protein levels determined by immunoblotting are shown for all transduced cell lines in Figure 1C.
HCK is hyperactive in MYD88-mutated cells, and its activation but not transcription is triggered by IL-6
Given the above findings, we next investigated the activation state of HCK in MYD88-mutated WM and DLBCL cell lines, and primary WM cells. By Phosflow analysis, HCK showed consistently high phosphorylation levels at the Tyr411 activation site in mutated MYD88 vs WT MYD88 cells (Figure 3A). Significantly higher levels of HCK Tyr411 phosphorylation were also observed in primary WM patient samples vs HD non-memory and memory B cells (Figure 3B). CXCR4 mutation status was available for 18 of 20 primary WM patient samples, and did not impact HCK Tyr411 phosphorylation (P = .65 for CXCR4 WT vs WHIM-mutated patients). Treatment of mutated MYD88 WM and DLBCL cell lines and primary WM LPCs with IL-6 augmented HCK activation, with a peak induction of Tyr411 phosphorylation at 5 minutes following IL-6 administration in mutated MYD88 cells (Figure 3C). In contrast, little or no effect on HCK Tyr411 phosphorylation was observed following IL-6 stimulation in MYD88WT cells. Knockdown of IL-6ST attenuated HCK activation in the presence or absence of IL-6, whereas transduction with a control vector had little or no impact on HCK activation in MYD88-mutated BCWM.1 cells (Figure 3D). Lastly, HCK transcription was not significantly altered following incubation with either IL-6 (1-10 ng/mL) or an IL-6 blocking antibody (1-10 μg/mL) for 2 hours in BCWM.1 and MWCL-1 cells using RT-PCR (data not shown).
HCK is hyperactive in MYD88-mutated cells, and its activation is triggered by IL-6. (A) Results of Phosflow analysis for phosphorylated (p) HCK (pHCKTyr411) in MYD88-mutated WM (BCWM.1 and MWCL-1) and ABC DLBCL (TMD-8 and OCI-Ly3) cells; MYD88 WT GCB DLBCL (OCI-Ly7 and OCI-Ly19), Ramos Burkitt, and RPMI-8226 myeloma cells. (B) Results of Phosflow analysis for pHCKTyr411 in primary LPCs (CD20+) from 3 representative MYD88 L265P expressing WM patients, as well as nonmemory (CD20+CD27−) and memory (CD20+CD27+) B cells from HDs. Percentage of cells gating for pHCKTyr411 expression are shown in (A-B). Histogram depicts the results of pHCKTyr411 expression in LPC derived from 20 WM patients, as well as nonmemory and memory B cells from 5 HDs. P < .01 for comparison of pHCK expression in WM LPC vs either HD nonmemory or memory B cells. The number of WM patients that were evaluated for LPC (open circles), as well as the number of healthy donors that were evaluated for nonmemory (open diamonds) and memory (open diamonds) B cells are shown. (C) pHCKTyr411 expression in the presence or absence of IL-6 (1 ng/mL) for 5 minutes in MYD88-mutated (BCWM.1, MWCL-1, TMD-8, HBL-1, and OCI-Ly3) and MYD88 WT (OCI-Ly7, OCI-Ly19, Ramos, and RPMI 8226) (top) cell lines, as well as primary WM LPCs. Peak pHCKTyr411 activation was determined by Phosflow analysis after 3 MYD88 mutated cell lines were cultured with IL-6 (1 ng/mL) for 2, 5, and 15 minutes (bottom). *P < .05; **P < .01; ***P < .001 vs untreated controls. (D) Phosflow analysis of pHCKTyr411 in BCWM.1 cells transduced with scrambled control vector or IL-6ST knockdown vector (shRNA2) in the presence or absence of 1 ng/mL of IL-6. Ab, antibody; MFI, mean fluorescence intensity; PBMCs, peripheral blood mononuclear cells; SSC, side scatter.
HCK is hyperactive in MYD88-mutated cells, and its activation is triggered by IL-6. (A) Results of Phosflow analysis for phosphorylated (p) HCK (pHCKTyr411) in MYD88-mutated WM (BCWM.1 and MWCL-1) and ABC DLBCL (TMD-8 and OCI-Ly3) cells; MYD88 WT GCB DLBCL (OCI-Ly7 and OCI-Ly19), Ramos Burkitt, and RPMI-8226 myeloma cells. (B) Results of Phosflow analysis for pHCKTyr411 in primary LPCs (CD20+) from 3 representative MYD88 L265P expressing WM patients, as well as nonmemory (CD20+CD27−) and memory (CD20+CD27+) B cells from HDs. Percentage of cells gating for pHCKTyr411 expression are shown in (A-B). Histogram depicts the results of pHCKTyr411 expression in LPC derived from 20 WM patients, as well as nonmemory and memory B cells from 5 HDs. P < .01 for comparison of pHCK expression in WM LPC vs either HD nonmemory or memory B cells. The number of WM patients that were evaluated for LPC (open circles), as well as the number of healthy donors that were evaluated for nonmemory (open diamonds) and memory (open diamonds) B cells are shown. (C) pHCKTyr411 expression in the presence or absence of IL-6 (1 ng/mL) for 5 minutes in MYD88-mutated (BCWM.1, MWCL-1, TMD-8, HBL-1, and OCI-Ly3) and MYD88 WT (OCI-Ly7, OCI-Ly19, Ramos, and RPMI 8226) (top) cell lines, as well as primary WM LPCs. Peak pHCKTyr411 activation was determined by Phosflow analysis after 3 MYD88 mutated cell lines were cultured with IL-6 (1 ng/mL) for 2, 5, and 15 minutes (bottom). *P < .05; **P < .01; ***P < .001 vs untreated controls. (D) Phosflow analysis of pHCKTyr411 in BCWM.1 cells transduced with scrambled control vector or IL-6ST knockdown vector (shRNA2) in the presence or absence of 1 ng/mL of IL-6. Ab, antibody; MFI, mean fluorescence intensity; PBMCs, peripheral blood mononuclear cells; SSC, side scatter.
HCK is a determinant of survival in MYD88-mutated cells
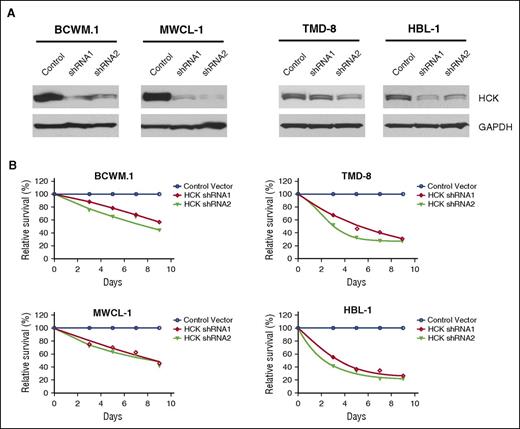
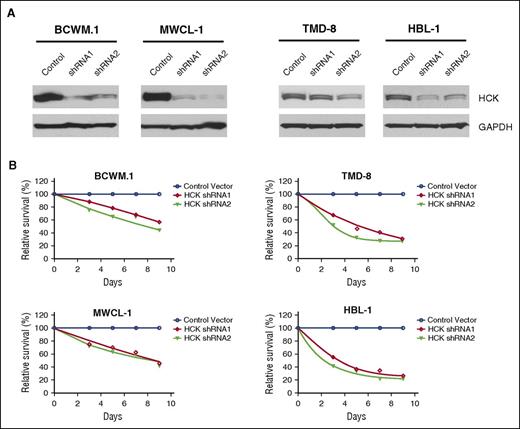
We next evaluated the impact of HCK expression on survival in MYD88-mutated BCWM.1 and MWCL-1 WM, and TMD-8 and HBL-1 ABC DLBCL cells. Successful knockdown of HCK was accomplished by lentiviral transduction using inducible shRNA vectors (Figure 4A). The results of these studies showed that knockdown of HCK in all 4 MYD88-mutated cell lines by either of the two shRNA vectors resulted in a sustained reduction in tumor cell viability over the 9-day evaluation period in contrast to cells transduced with a control vector (Figure 4B).
HCK is a determinant of survival in MYD88-mutated cells. (A) HCK protein levels determined by western blot analysis, and (B) survival determined by AlamarBlue cell viability assay over a 9-day evaluation period in MYD88-mutated BCWM.1 and MWCL-1 WM cells (left), and TMD-8 and HBL-1 ABC DLBCL cells (right) following transduction with inducible HCK knockdown (shRNA1 and shRNA2) or scrambled control vectors. Mean of 2 independent experiments is depicted for time points.
HCK is a determinant of survival in MYD88-mutated cells. (A) HCK protein levels determined by western blot analysis, and (B) survival determined by AlamarBlue cell viability assay over a 9-day evaluation period in MYD88-mutated BCWM.1 and MWCL-1 WM cells (left), and TMD-8 and HBL-1 ABC DLBCL cells (right) following transduction with inducible HCK knockdown (shRNA1 and shRNA2) or scrambled control vectors. Mean of 2 independent experiments is depicted for time points.
HCK triggers pro-survival signaling in MYD88-mutated WM cells
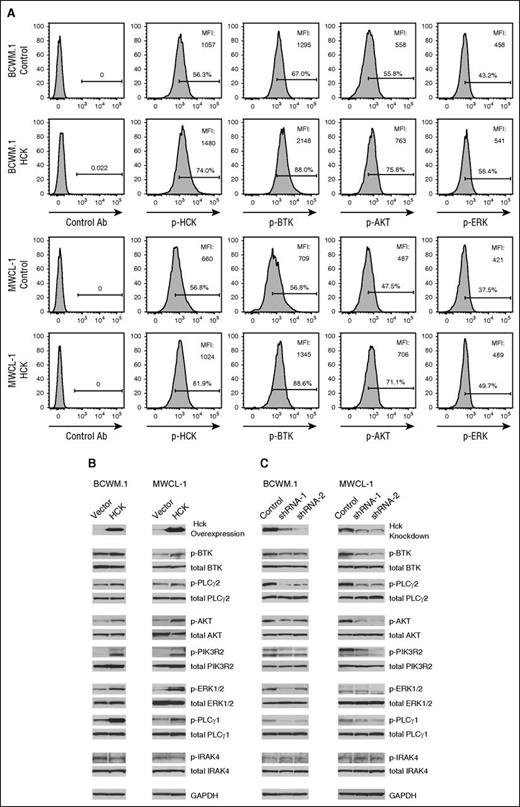
In view of the above findings, we next interrogated HCK-dependent survival signaling in MYD88-mutated WM cells. We focused our efforts on PI3K/AKT, mitogen-activated protein kinase (MAPK), and BTK signaling pathways given their established importance in WM survival, as well as previous work implicating HCK as an upstream regulator for their signaling.21-24 Transduction of HCK in IL-6–producing BCWM.1 and MWCL-1 cells led to higher HCK protein levels by western blot analysis and detection of activated HCK (Tyr411) by Phosflow analysis (Figure 5A-B). Transduction of HCK in BCWM.1 and MWCL-1 cells also triggered PI3K/AKT (pPIK3R2 and pAKT), MAPK (pPLCγ1 and pERK1/2), and BTK (pBTK and pPLCγ2) signaling (Figure 5B), whereas knockdown of HCK in BCWM.1 and MWCL-1 cells showed a reciprocal pattern, with diminished PI3K/AKT, MAPK, and BTK signaling (Figure 5C). IRAK4 activation was not impacted by either HCK over-expression or knockdown. Total protein levels for these signaling molecules and GAPDH remained unchanged in these experiments.
HCK triggers pro-survival signaling in MYD88-mutated WM cells. (A) Phosflow analysis for pHCKTyr411, pBTK, pAKT, and pERK in scramble control vector (top) or HCK transduced MYD88 mutated BCWM.1 and MWCL-1 cells (bottom). (B) Impact of HCK over-expression or (C) HCK knockdown on PI3K/AKT (PIK3R2 and AKT), MAPK (PLCγ1 and ERK1/2), BTK (BTK and PLCγ2), and IRAK4 signaling in BCWM.1 and MWCL-1 cells using antibodies to detect phospho-specific and total protein expression by immunoblotting. GAPDH protein levels were determined for control purposes in all experiments.
HCK triggers pro-survival signaling in MYD88-mutated WM cells. (A) Phosflow analysis for pHCKTyr411, pBTK, pAKT, and pERK in scramble control vector (top) or HCK transduced MYD88 mutated BCWM.1 and MWCL-1 cells (bottom). (B) Impact of HCK over-expression or (C) HCK knockdown on PI3K/AKT (PIK3R2 and AKT), MAPK (PLCγ1 and ERK1/2), BTK (BTK and PLCγ2), and IRAK4 signaling in BCWM.1 and MWCL-1 cells using antibodies to detect phospho-specific and total protein expression by immunoblotting. GAPDH protein levels were determined for control purposes in all experiments.
Ibrutinib binds to the ATP-binding pocket of HCK and blocks ATP binding
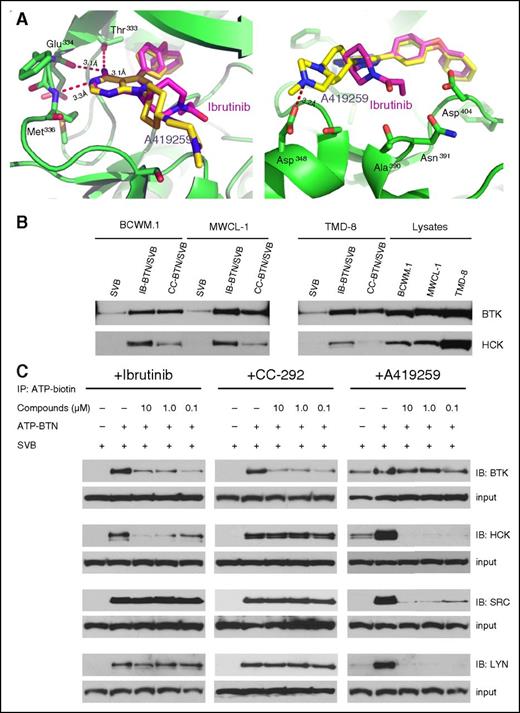
Kinase selectivity profiling has established that, in addition to being a covalent inhibitor of BTK, ibrutinib is also a potent noncovalent inhibitor of several SRC family members including HCK, YES, and LCK.10 We focused our attention on investigating the potential functional significance of HCK to ibrutinib’s pharmacology due to its structural similarity with A419259, an HCK inhibitor with established activity in murine tumor models.15 Indeed, both ibrutinib and A419259 were developed based on the 4-amino-5,7-substituted pyrrolopyrimidine scaffold of the classical SRC-family inhibitor: PP1.25 To create a model for how ibrutinib might bind to HCK, we performed molecular docking studies of ibrutinib into the co-crystal structure of HCK with A419259 (RK-20449) (PDB 3VS3)26 (Figure 6A). As expected, the docking study predicts that ibrutinib recognizes the ATP-binding pocket of HCK with an almost identical pose as A419259 with a calculated affinity energy (ΔG) of −10.5 kcal/mol. Similar to A419259, ibrutinib’s 4-amino group, forms an hydrogen-bond to the carbonyl groups of the gatekeeper residue Thr333 (Thr338 based on c-SRC numbering)15 and also with Glu334 of HCK; its 3-nitrogen atom, as an hydrogen-bond acceptor, interacts with the backbone amino group of Met336 of HCK (Figure 6A). The 4-phenoxyphenyl substituent at 5-position of ibrutinib, which is also identical to the 5-substituent of A419259, extends into the inner ATP-hydrophobic pocket of HCK. The pyrrolidine group at the 7-position of ibrutinib, the only distinctive substituent related to A419259, is predicted to interact with Ala390, Asn391, or Asp404 of HCK, whereas the 7-substituent of A419259 protrudes outward the ATP pocket and interacts with Asp348 (Figure 6A).
Ibrutinib binds to the ATP-binding pocket of HCK and blocks ATP binding. (A) Docking model of the ibrutinib (purple stick) aligned with the co-crystal structure of HCK (green ribbon)-A419259 (yellow stick). The hydrogen bonds (depicted as red dashed lines) between the aminopyrimidine moiety of ibrutinib and the hinge region of HCK are indicated. Docking studies showed that ibrutinib bound to the ATP-binding pocket of HCK with calculated affinity energy (ΔG) of −10.5 kcal/mol. (B) Results from pull-down experiments using SVB, IB-BTN, and CC-292 (CC-BTN) to detect BTK and HCK binding in MYD88-mutated BCWM.1, MWCL-1, and TMD-8 cells. (C) Results from kinase active-site inhibition assays utilizing an ATP-BTN probe that was used to pull down active kinases in the presence of ibrutinib, CC-292, or A419259 in lysates from BCWM.1 WM cells. ATP-BTN, ATP-biotin; IB-BTN, biotinylated ibrutinib; SVB, streptavidin beads.
Ibrutinib binds to the ATP-binding pocket of HCK and blocks ATP binding. (A) Docking model of the ibrutinib (purple stick) aligned with the co-crystal structure of HCK (green ribbon)-A419259 (yellow stick). The hydrogen bonds (depicted as red dashed lines) between the aminopyrimidine moiety of ibrutinib and the hinge region of HCK are indicated. Docking studies showed that ibrutinib bound to the ATP-binding pocket of HCK with calculated affinity energy (ΔG) of −10.5 kcal/mol. (B) Results from pull-down experiments using SVB, IB-BTN, and CC-292 (CC-BTN) to detect BTK and HCK binding in MYD88-mutated BCWM.1, MWCL-1, and TMD-8 cells. (C) Results from kinase active-site inhibition assays utilizing an ATP-BTN probe that was used to pull down active kinases in the presence of ibrutinib, CC-292, or A419259 in lysates from BCWM.1 WM cells. ATP-BTN, ATP-biotin; IB-BTN, biotinylated ibrutinib; SVB, streptavidin beads.
To confirm HCK target engagement in cells by ibrutinib, we synthesized a biotin-modified version of ibrutinib. For comparative purposes, we also prepared a biotin-modified version of CC-292, a pyrimidine-based covalent BTK inhibitor.27 Both ibrutinib and CC-292 use an acrylamide-moeity to form a covalent bond to Cys481 and are potent kinase inhibition of BTK (reported half maximal inhibitory concentration [IC50] ≤ 0.5 nM).10,27 Ibrutinib reversibly inhibits the kinase activity of HCK with an IC50 of 3.7 nM, whereas CC-292 is a very weak HCK inhibitor with an IC50 of 14.6 μM, which is well above the observed physiological concentrations of this drug.10,27 As expected, biotinylated ibrutinib and CC-292 pulled down BTK in mutated MYD88 expressing BCWM.1, MWCL-1, and TMD-8 cells, demonstrating their ability to directly bind to BTK (Figure 6B). Biotinylated ibrutinib, but not CC-292 pulled down HCK in MYD88-mutated BCWM.1, MWCL-1, and TMD-8 cells, thereby confirming binding of ibrutinib to HCK (Figure 6B).
To confirm target engagement in living cells, we performed KiNativ profiling where the ability of inhibitors to protect kinases from subsequent labeling with a reactive ATP-biotin probe is determined.28 Living cells were treated with either ibrutinib, CC-292, or A419259, followed by lysis treatment with ATP-biotin and western blotting for BTK, HCK, and other SRC family kinases. Consistent with the biochemical kinase assays, ibrutinib and A419259 but not CC-292 blocked ATP binding to HCK in a dose-dependent manner, whereas ibrutinib and CC-292 but not A419259 blocked ATP binding to BTK in BCWM.1 WM cells. Consistent with its known activity, A419259 also blocked ATP binding to the SRC family members SRC and LYN. Conversely, ibrutinib and CC-292 did not block ATP binding to either SRC or LYN (Figure 6C).
HCK activity is blocked by ibrutinib and impacts survival of MYD88-mutated WM cells
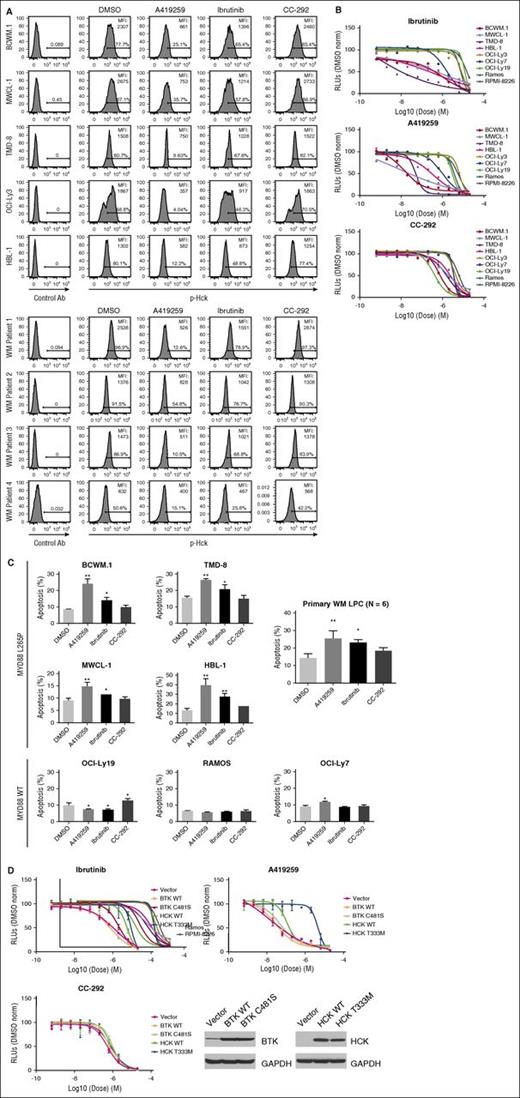
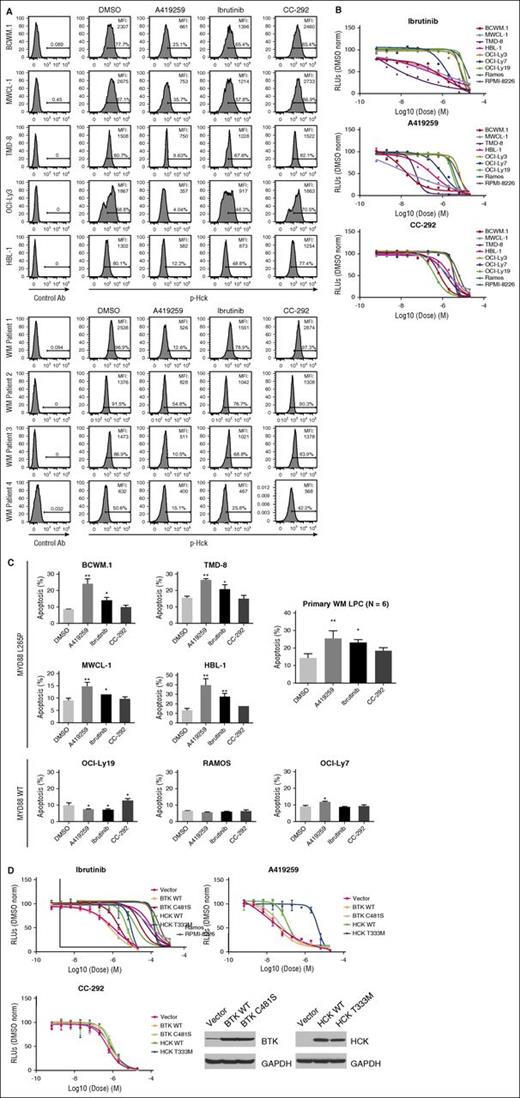
To assess the impact of ibrutinib and A419259 on HCK-related activity in MYD88-mutated WM and ABC DLBCL tumor cells, we performed Phosflow studies and assessed changes in HCK activation (Tyr411). We also evaluated tumor-cell viability by the CellTiter-Glo Luminescent Cell Viability Assay, and apoptosis by PI and Annexin V staining to determine if HCK inhibition contributed to WM cell death. We observed that ibrutinib, and A419259 even more so, reduced HCK Tyr411 phosphorylation in MYD88 L265P expressing WM and ABC-DLBCL cell lines, as well as primary WM LPC. In contrast, CC-292 had little or no impact on HCK Tyr411 phosphorylation (Figure 7A). Decreased dose-dependent viability was also observed for ibrutinib and more so for A419259 that was more pronounced in mutated MYD88 vs MYD88WT cells (Figure 7B). In contrast, the BTK inhibitor CC-292 that lacks HCK kinase inhibition showed higher half maximal effective concentration (>1 log-fold) for the mutated MYD88 cell lines, with the exception of OCI-Ly3 cells that carry a NF-κB activating CARD11 mutation (Figure 7B).5 Increased apoptotic changes were also observed in the mutated MYD88 cell lines and primary WM cells that showed decreased viability following ibrutinib, and A419259 even more so. In contrast, CC-292 had little or no impact on apoptosis in mutated MYD88 cells (Figure 7C). Also, we observed little or no apoptotic activity for ibrutinib, A419259, or CC-292 apoptosis in MYD88WT cell lines or HD B cells (data not shown). To investigate whether ibrutinib or A419259-induced killing of WM cells was a consequence of BTK and/or HCK inhibition, we transduced MYD88L265P expressing BCWM.1 WM cells with a lentiviral control vector or vectors expressing WT BTK; BTK with a mutation of the cysteine required for irreversible inhibition (BTKC481S); WT HCK; or HCK with the gatekeeper mutation Thr333 (Thr338 based on c-SRC numbering).15 Transduction of BCWM.1 WM cells with the BTK cysteine mutant (BTKC481S) but not the vector control or WT BTK, resulted in decreased ibrutinib and CC-292–mediated killing of BCWM.1 WM cells (<1 log-fold shift) (Figure 7D). In contrast, treatment with HCK inhibitor A419259 showed no survival change vs vector control transduced cells (Figure 7D).
HCK activity is blocked by ibrutinib and impacts the survival of MYD88-mutated WM cells. (A) Phosflow analysis showing changes in pHCKTyr411 following treatment of MYD88-mutated WM (BCWM.1 and MWCL-1) and ABC DLBCL (TMD-8, OCI-Ly3, and HBL-1) cells and MYD88-mutated primary LPCs from 4 WM patients with 0.5 μM of A419259, ibrutinib, or CC-292 for 30 minutes. (B) Dose-dependent survival determined by CellTiter-Glo Luminescent Cell Viability Assay for mutated (BCWM.1, MWCL-1, TMD-8, HBL-1, and OCI-LY3) and WT (OCI-LY7, OCI-LY19, Ramos, and RPMI 8226) MYD88 cells following treatment with ibrutinib (top), A419259 (middle), or CC-292 (bottom) for 72 hours. (C) Apoptotic changes using PI and Annexin V (FITC-A) staining following treatment of mutated and WT MYD88 cell lines, and primary LPCs from 3 WM patients with 0.5 μM of A419259, ibrutinib, or CC-292 for 18 hours. DMSO denotes vehicle control only treated cells. Cell line results are from experiments performed in triplicate. Primary LPC data are from results obtained from 6 WM patients. *P ≤ .05 and **P ≤ .01 vs DMSO controls. (D) Dose-dependent tumor cell survival of MYD88-mutated BCWM.1 cells transduced with control vector or vectors expressing WT BTK (BTK WT); BTK-expressing mutated site for ibrutinib binding (BTK C481S); WT HCK (HCK WT); or HCK-expressing the gatekeeper mutation (HCKT333M; HCKT338M based on c-SRC numbering)15 and treated with ibrutinib, A419259, or CC-292. Protein levels following transduction with control, WT, or mutated BTK or HCK vectors are also shown at the bottom of (D). FITC-A, fluorescein isothiocyanate A; RLU, relative luminescence units.
HCK activity is blocked by ibrutinib and impacts the survival of MYD88-mutated WM cells. (A) Phosflow analysis showing changes in pHCKTyr411 following treatment of MYD88-mutated WM (BCWM.1 and MWCL-1) and ABC DLBCL (TMD-8, OCI-Ly3, and HBL-1) cells and MYD88-mutated primary LPCs from 4 WM patients with 0.5 μM of A419259, ibrutinib, or CC-292 for 30 minutes. (B) Dose-dependent survival determined by CellTiter-Glo Luminescent Cell Viability Assay for mutated (BCWM.1, MWCL-1, TMD-8, HBL-1, and OCI-LY3) and WT (OCI-LY7, OCI-LY19, Ramos, and RPMI 8226) MYD88 cells following treatment with ibrutinib (top), A419259 (middle), or CC-292 (bottom) for 72 hours. (C) Apoptotic changes using PI and Annexin V (FITC-A) staining following treatment of mutated and WT MYD88 cell lines, and primary LPCs from 3 WM patients with 0.5 μM of A419259, ibrutinib, or CC-292 for 18 hours. DMSO denotes vehicle control only treated cells. Cell line results are from experiments performed in triplicate. Primary LPC data are from results obtained from 6 WM patients. *P ≤ .05 and **P ≤ .01 vs DMSO controls. (D) Dose-dependent tumor cell survival of MYD88-mutated BCWM.1 cells transduced with control vector or vectors expressing WT BTK (BTK WT); BTK-expressing mutated site for ibrutinib binding (BTK C481S); WT HCK (HCK WT); or HCK-expressing the gatekeeper mutation (HCKT333M; HCKT338M based on c-SRC numbering)15 and treated with ibrutinib, A419259, or CC-292. Protein levels following transduction with control, WT, or mutated BTK or HCK vectors are also shown at the bottom of (D). FITC-A, fluorescein isothiocyanate A; RLU, relative luminescence units.
Transduction of BCWM.1 WM cells with the HCK gatekeeper mutant (HCKT333M; HCKT338M based on c-SRC numbering15 ) resulted in a >2 log-fold decrease in both ibrutinib and A419259-mediated tumor cell killing. Over-expression of WT HCK also resulted in a 1 log-fold decrease in both ibrutinib and A419259-mediated tumor cell killing when compared with control vector transduced BCWM.1 WM cells (Figure 7D). No substantial change in tumor cell killing following CC-292 treatment was observed in BCWM.1 cells transduced to express HCKT333M or WT HCK (Figure 7D).
Discussion
We sought to clarify the contribution of non-BTK target(s) to the activity of ibrutinib in MYD88-mutated diseases such as WM. We focused this study on HCK, a member of the SRC family of kinases. HCK is over-expressed at early stages of B-cell development, and its transcriptional regulation is suppressed with terminal B-cell differentiation.29,30 Using gene expression profiling, Gutiérrez et al11 previously reported that HCK was highly over-expressed in malignant LPC taken from WM patients, although the genetic basis for this observation remained unclear. In this study, we observed that HCK was highly expressed in MYD88-mutated primary WM cells, WM, and ABC-DLBCL cell lines, but was absent or expressed at low levels in HD B cells, as well as MYD88WT cell lines. Furthermore, we observed that over-expression of the MYD88L265P protein itself markedly induced HCK transcription across multiple B-cell lines, including both MYD88-mutated and WT cell lines. It is not likely that HCK transcription was triggered by MYD88-triggered IL-6, because exogenous treatment of MYD88-mutated WM cells with IL-6 or an IL-6 antibody did not directly impact HCK transcription. In addition, CXCR4 mutations that are found in up to 40% of WM patients neither impacted HCK transcription or activation in primary WM cells, nor did we observe HCK transactivation in previous studies with MYD88-mutated BCWM.1 and MWCL-1 cells that were transduced with CXCR4 WHIM-mutated receptors and stimulated with CXCL12 (unpublished observations).31,32 Our findings therefore provide a genomic explanation for HCK over-expression in WM, and suggest that mutated MYD88 enforces high transcription levels of HCK. NF-κB, activator protein 1, and cAMP-response element binding sites are present on the promoter of HCK, and their transactivation is known to be triggered by WT MYD88 in response to toll-like receptor stimulation.31-35 Studies to delineate the precise signaling cascade(s) by which mutated MYD88 transduces HCK are therefore needed, although the constitutive activation of NF-κB by mutated MYD88 is a strong suspect in WM and ABC DLBCL cells, as well as activator protein 1 in ABC DLBCL.1,5,36 The BCR and other pro-survival pathways may also contribute to HCK activation and warrant further investigation.
The findings also suggest an important role for IL-6 in triggering HCK activity in WM, and possibly other MYD88-mutated diseases. We show that both IL-6 and IL-6R are highly expressed in WM disease, and that MYD88 L265P itself induces IL-6 transcription. Ngo et al5 showed that HCK and IL-6 were among 285 genes whose transcription was downregulated upon knockdown of MYD88 in HBL-1 ABC-DLBCL cells that express L265P as a heterozygous mutation. The role of IL-6 as an activator of pHCK through IL-6R/IL-6ST has previously been reported, and appears dependent on an acidic domain (amino acids 771-811) of IL-6ST.12,13 IL-6 is highly transcribed in WM and ABC-DLBCL tumors.11,37,38 IL-6 is also secreted by the BM microenvironment in response to WM cell adhesion.14 We show in these studies that HCK is hyper-activated in mutated MYD88 primary WM cells as well as WM and ABC-DLBCL cell lines, and that exogenous IL-6 induced its activation, whereas knockdown of IL-6ST attenuated HCK activation. HCK was also found to induce PI3K/AKT, MAPK/ERK, and BTK signaling, providing for the first time evidence that mutated MYD88 can trigger pro-survival signaling beyond its known activation of NF-κB by eliciting HCK transcription and activation. The transactivation of BTK by HCK is also noteworthy. Cheng et al previously described that BTK could bind to HCK through SH3-mediated interactions.21 In previous work, we described that the activated-form BTK was complexed with MYD88 in MYD88-mutated cells, although the mechanism for BTK activation in this context remained unclear. Our findings offer a mechanistic explanation for BTK activation in MYD88-mutated WM and ABC DLCBL cells.
A key finding of this study was the recognition of HCK as a novel therapeutic target for WM, and possibly other mutated MYD88 diseases. Kinase selectivity profiling had previously implicated HCK as a potential target of ibrutinib with an IC50 of 3.7 nM, a dose level close to the IC50 of BTK (0.5 nM) and well within the pharmacologically achievable levels of ibrutinib.10,39 We provide definitive evidence in this report that HCK is a target of ibrutinib using docking models, direct pull-down experiments with biotinylated ibrutinib, cellular target engagement, and cellular rescue with inhibitor-resistant mutant proteins. We also observed that treatment of mutated MYD88 WM and ABC-DLBCL cells with ibrutinib, or a preclinical HCK inhibitor (A419259) attenuated HCK activation, whereas over-expression of WT HCK and more so HCK bearing a mutated gatekeeper (HCKT333M; HCKT338M based on c-SRC numbering)15 reduced the activity of both of these compounds in MYD88-mutated cells. The attenuation of MYD88-directed HCK activity may provide mechanistic insights into the high response activity observed in MYD88-mutated WM and ABC-DLBCL patients undergoing ibrutinib therapy.4,9 It also remains possible that mutations in the gatekeeper site of HCK could contribute to ibrutinib resistance, akin to that observed with BTKC481S mutations in chronic lymphocytic leukemia patients, and warrant examination.40 Finally, the potential to develop more potent HCK inhibitors is suggested by these findings. Although ibrutinib suppressed HCK activity, higher levels of HCK suppression were observed with A419259, and were associated with greater levels of apoptosis in MYD88-mutated WM and ABC-DLBCL cells. Several scaffolds for targeted HCK inhibition are in development based on prior work implicating HCK in the pathogenesis of several solid and hematologic malignancies, as well as productive HIV infection.41,42 In addition to inhibiting other SRC family members, dasatinib also shows potent inhibition of HCK and may be active in diseases dependent on mutated MYD88.43
In conclusion, our findings suggest that HCK is a downstream target of mutated MYD88, is activated by IL-6, and triggers pro-survival signaling including PI3K/AKT, MAPK/ERK, and BTK in MYD88-mutated cells. HCK is also a highly relevant target of ibrutinib, and represents a novel focus for therapeutic development in WM and possibly other diseases driven by mutated MYD88.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge the generous support of Peter Bing, the International Waldenström’s Macroglobulinemia Foundation, the Leukemia and Lymphoma Society, the Edward and Linda Nelson Fund for WM Research, the Bauman Family Trust, the Tannenhauser Family Foundation, and the WM patients who provided samples for these studies. The authors also acknowledge the kind gift of Thomas Smithgall for providing the HCK inhibitor-resistant mutant construct used in these studies.
Authorship
Contribution: G.Y., S.J.B., L.T., N.G., and S.P.T. conceived and designed the experiments; G.Y. and S.P.T. wrote the manuscript; G.Y., Z.R.H., and S.P.T. performed the data analysis; X.L. and N.T. performed PCR-based sequencing studies; G.Y., X.L., and J.C. performed transduction experiments; X.L. and J.G.C. performed western blot studies; G.Y. and J.C. performed Phosflow studies; L.T., S.J.B., and N.G. performed docking studies; L.X., X.L., and C.J.P. prepared samples; S.P.T., J.R.B., and J.J.C. provided patient care, and obtained consent and samples; W.Z., X.Z., and S.L. provided biotinylated ibrutinib; and P.C. provided BTK-C481S and HCK-T333M vectors for transduction experiments.
Conflict-of-interest disclosure: S.P.T. and J.R.B. have received research funding, consulting fees, and/or honoraria from Pharmacyclics Inc., and Janssen Oncology Inc. The remaining authors declare no competing financial interests.
Correspondence: Steven P. Treon, Dana Farber Cancer Institute and Harvard Medical School, 450 Brookline Ave, M546, Boston, MA 02215; e-mail: steven_treon@dfci.harvard.edu.