Abstract
Despite recent advances in the field of allogeneic hematopoietic stem cell transplantation (HSCT), viral infections are still a major complication during the period of immune suppression that follows the procedure. Adoptive transfer of donor-derived virus-specific cytotoxic T cells (VSTs) is a strategy to rapidly restore virus-specific immunity to prevent or treat viral diseases after HSCT. Early proof of principle studies demonstrated that the administration of donor-derived T cells specific for cytomegalovirus or Epstein-Barr virus (EBV) could effectively restore virus-specific immunity and control viral infections. Subsequent studies using different expansion or direct selection techniques have shown that donor-derived VSTs confer protection in vivo after adoptive transfer in 70% to 90% of recipients. Because a major cause of failure is lack of immunity to the infecting virus in a naïve donor, more recent studies have infused closely matched third-party VSTs and reported response rates of 60% to 70%. Current efforts have focused on broadening the applicability of this approach by: (1) extending the number of viral antigens being targeted, (2) simplifying manufacture, (3) exploring strategies for recipients of virus-naïve donor grafts, and (4) developing and optimizing “off the shelf” approaches.
Introduction
Viral infections remain a leading cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT).1 The use of prophylactic pharmacotherapy is effective in reducing the risk for some viral infections, but therapeutic options for breakthrough infections are complicated by toxicities, and for many viral infections there are limited/no effective prophylactic or therapeutic pharmacotherapies.2 T-cell reconstitution is a key requirement for effective antiviral control following HSCT, and factors that influence the speed of T-cell recovery also impact the risk of viral infection in this period.3 The use of donor lymphocyte infusions derived from seropositive stem cell donors is an effective salvage therapy for viral infections in HSCT recipients prior to T-cell recovery, but the risk of potentially severe graft-versus-host disease (GVHD) is a concern.4,5 Depletion of specific T-cell subsets such as naïve T cells from the infused HSCT product may preserve antiviral immunity while depleting alloreactive cells,6 and it is also possible to deplete alloreactive populations from donor lymphocyte infusions products to produce antiviral activity in the absence of GVHD, or to include a suicide gene as a safety switch in an alloreplete product.7,8
A more specific approach to expedite virus-specific T-cell (VST) reconstitution is adoptive transfer of donor-derived VSTs, and this strategy has been successfully applied over the last 2 decades in many centers to prevent and treat viral infections.9-12 Development of VST therapy to broader applicability has been facilitated by several advances in immunobiology, including: (1) the knowledge of conserved T-cell epitopes for various pathogens,13-16 (2) improvements in ex vivo culture methodologies for the generation of T cells and antigen-presenting cells (APCs),16-18 and (3) rapid assays to evaluate the effector function of VSTs.16
In this study, we summarize the methodologies used in generating donor-derived VSTs, review the results of clinical trials using VST therapies after HSCT, and discuss how recent manufacturing improvements to simplify the VST generation process and the use of third-party banks now allow testing of this strategy in later phase clinical trials.
Ground rules for T-cell manufacturing
Antigen selection
The ex vivo generation and expansion of VSTs for clinical use requires: (1) a defined immunogenic antigen, and (2) an APC that can effectively present antigen to the T cells with appropriate costimulatory signals. Hence, for a given virus, it is important to know which viral antigens are immunodominant and induce protective T cells in vivo. For some latent viruses (eg, cytomegalovirus [CMV] and Epstein-Barr virus [EBV]), the immunodominant antigens expressed at different stages of infection have been well defined,13,15 but for others such as adenovirus (ADV), human herpesvirus 6 (HHV6), and BK virus, the appropriate target antigens had to be identified to design adoptive immunotherapy studies.16,19,20 This process has been simplified by the increasing availability of bio-informatic tools that enable the effective mapping of viral epitopes recognized by both CD4+ and CD8+ T cells.
Antigen presentation
Defined immunogenic antigens must be presented utilizing an APC that expresses major histocompatibility complex antigens to present virus antigen-derived peptides, as well as costimulatory molecules sufficient to induce T-cell activation and expansion. The choice of APC also depends on the type of viral antigen to be used and the proposed method of delivery. Examples of antigens used to manufacture VSTs include whole virions, specific viral genes, whole proteins, or peptides. APCs used include fibroblasts, dendritic cells (DCs), monocytes, B cells, and artificial K562-based cells. Specific examples include:
Whole virus or viral lysate to expand VSTs. The use of the entire viral antigen enables the generation of a broad CD8+ as well as CD4+ T-cell response. CMV lysate or antigen have both been used to infect fibroblasts or to pulse DCs to ex vivo expand CMV-specific T cells.11,12 Similarly, initial studies used B cells infected with the B95-8 laboratory strain of EBV to generate EBV-infected lymphoblastoid cell lines (LCLs), which are potent APCs, to ex vivo expand polyclonal and polyfunctional EBV-specific T cells.9,21 However, the use of viral lysate or LCLs containing an infectious virus makes it more challenging to transition an approach to late phase trials given the potential infection risk.
Whole proteins. In some cases, a whole viral protein has been used. In this case, the protein must be universally expressed and immunogenic. Examples include the use of the CMV-pp65 protein to manufacture CMV-specific T cells22 or the use of the Epstein-Barr nuclear antigen-1 (EBNA-1) protein to expand EBNA-1–specific T cells for EBV-associated posttransplant lymphoproliferative disease (PTLD).23
Gene-modified APC (viral vectors and plasmids). Another approach is to genetically modify APCs with ADV vectors encoding viral antigens such as CMV-pp65, or EBV latent membrane protein 2 (LMP2) to expand VSTs. ADV vectors (with or without a transgene) also effectively expand T cells recognizing the ADV virion proteins hexon and penton, expressed by the vector itself. Further, transduction of LCL with an ADV vector not only expands ADV hexon/penton-specific T cells but also expands T cells specific for the latent and early lytic cycle EBV proteins expressed by the LCL.24 As an alternative to viral vectors, plasmids expressing viral antigens derived from multiple viruses have also been used to transfect DCs to stimulate multivirus-specific T cells for clinical use.25
Peptides and peptide mixtures. Several groups have explored the use of individual HLA-restricted peptides/epitopes such as the A2-restricted CMV-pp65 epitope NLV to stimulate VSTs.26 Concerns with this approach are that targeting a single epitope will result in escape mutants and that it is restricted to patients with specific HLA types. However, the more recent availability of overlapping peptide pools or pepmixes where overlapping peptide libraries represent the entire protein sequences of the target viral antigens has provided a readily available and good manufacturing practice (GMP)-compliant source of viral antigen(s), which have been validated in several preclinical and clinical studies.16,18,20,27,28 Pepmixes are now the most widely used source of viral antigen in current clinical trials.
Artificial APC. Autologous APCs may be limited, especially if large numbers of VSTs are required or to help priming of the virus-specific response in vitro (eg, when the donor is seronegative) is required. Several groups have explored the use of artificial APCs. For example, a novel artificial antigen-presenting complex (KATpx) was developed for the expansion of VSTs for clinical use. Using this approach, viral pepmixes were presented by APCs and these peptide-loaded cells were cocultured with irradiated HLA-negative K562 cells genetically modified to express CD80, CD83, CD86, and 4-1BBL (K562cs) that provided complementary costimulation in trans to effectively stimulate and expand VSTs.29 An alternative strategy to obtain larger numbers of APCs is to use the stem cell product as a source of cells for APC production.30
Expansion strategies
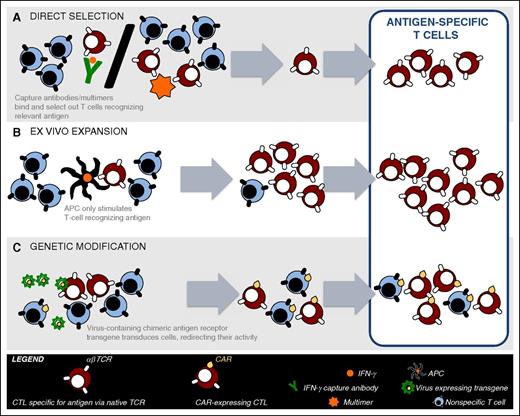
The manufacture of VST products for clinical use requires the selection of VSTs and the exclusion of alloreactive T cells using a GMP-compliant approach. Initial studies used complex methodologies requiring significant ex vivo expansion and in some cases cloning.9,12 In order to make VSTs more broadly available, recent studies have focused on rapid manufacturing strategies including: (1) direct selection of donor-derived VSTs with multimers or γ capture, and (2) stimulation and brief ex vivo expansion of donor T cells (Figure 1). The advantage and disadvantages of each approach, as well as the use of third-party T cells, are summarized in Table 1.
VST manufacturing strategies. Donor blood is drawn and PBMCs are then manipulated using different approaches for the manufacture of VSTs for clinical use. (A) Direct selection utilizes either: (1) multimers specific for a virus-derived peptide in the setting of a class-I HLA molecule, or (2) column selection where T cells are stimulated with viral antigen followed by selection of IFN-γ or CD154–expressing T cells using antibody coated immunomagnetic beads. (B) Ex vivo T-cell expansion requires the in vitro stimulation and expansion of T cells using APCs pulsed, infected, or transfected with viral peptide(s)/protein(s), viral lysate, or viral vectors/plasmids, respectively. (C) Genetic modification requires the gene transfer of high affinity VST receptors or chimeric-antigen receptors to redirect specificity of T cells to viral targets. CAR, chimeric antigen receptor.
VST manufacturing strategies. Donor blood is drawn and PBMCs are then manipulated using different approaches for the manufacture of VSTs for clinical use. (A) Direct selection utilizes either: (1) multimers specific for a virus-derived peptide in the setting of a class-I HLA molecule, or (2) column selection where T cells are stimulated with viral antigen followed by selection of IFN-γ or CD154–expressing T cells using antibody coated immunomagnetic beads. (B) Ex vivo T-cell expansion requires the in vitro stimulation and expansion of T cells using APCs pulsed, infected, or transfected with viral peptide(s)/protein(s), viral lysate, or viral vectors/plasmids, respectively. (C) Genetic modification requires the gene transfer of high affinity VST receptors or chimeric-antigen receptors to redirect specificity of T cells to viral targets. CAR, chimeric antigen receptor.
Ex vivo expansion of antigen-specific VSTs.
The original and most widely used methodology for VST generation is stimulation and ex vivo culture to expand T cells targeting one or multiple viruses in a single product. Ex vivo expansion has several advantages over direct T-cell selection, including the generation of polyclonal T cells, and the expansion of T cells to clinically useful numbers from a small starting volume of blood.16 These advantages may come at the expense of the required T-cell manufacture time, which can vary from 10 days to >3 months. Although T-cell exhaustion is a concern with the use of prolonged ex vivo culture and expansion methods, clinical trials utilizing gene-marked VSTs post-HSCT have demonstrated long-term (>10 years) persistence of the cells.31 Moreover, studies have shown that ex vivo culturing with virus-specific stimuli reduces alloreactivity in vitro, and any residual alloreactivity detected has not correlated with an increased risk of clinically significant GVHD.32 Over the past few years, there have been a number of advances, including the availability of overlapping peptide pools as a source of antigen, and the development of optimized vessels and culture conditions to rapidly expand T cells that have simplified this process while preserving viral specificity and optimal phenotype.16,17,20 This is relevant as T-cell products derived from CD62L+/CD45RA− central memory T cells have superior persistence in vivo following adoptive transfer.33 Another approach relied on the selection of T cells specific for viral and fungal pathogens based on activation-dependent expression of CD154, which enabled the generation of single- and multi-pathogen–specific T-cell lines in 14 days even if the precursor frequency was low.34
Direct selection of donor T cells.
One rapid selection strategy is direct isolation of VSTs from donor peripheral blood using peptide-HLA multimers, which isolate T cells based on the ability of their receptor to bind to a complex of synthetic peptide-loaded recombinant HLA molecules. This process is restricted by HLA type and necessitates knowing which epitopes are immunodominant. The donor also needs to have a high frequency of T cells specific for the peptide used. Consequently, the broadest application of this approach has been for CMV and EBV, where the frequency of VSTs is usually high enough in seropositive donors to enable selection. In addition, multimers are most readily made with class I HLA antigens, so the duration of an immune response following adoptive transfer may be limited in the absence of CD4+ T cells. Recently, researchers have evaluated streptamers, that is, multimers in which the HLA molecule-peptide and antigen-specific T-cell receptor (TCR) binding is reversible by a competitor molecule that causes the streptamer to monomerize.35 Streptamer selection has been used in a large European study, which has so far only been reported in abstract form.36
An alternate strategy, interferon-γ (IFN-γ) capture is based on secretion of IFN-γ by VSTs after short-term stimulation with antigen; IFN-γ–secreting populations are then captured by labeling with an anti–IFN-γ monoclonal antibody conjugated to a leukocyte-specific (CD45) antibody, followed by magnetic selection. This strategy selects both CD4+ and CD8+ antigen-specific T cells in an HLA unrestricted manner, and has been used in trials to treat ADV, CMV, and EBV infections in the post-HSCT setting.23,37,38
Both of these direct selection strategies have the advantage of rapid manufacturing time, but also generally requires that the donor have an additional leukapheresis in order to collect sufficient cells for clinical use, which can be impractical in the unrelated-donor setting. Additionally, this approach necessitates that VSTs be readily detectable in the peripheral blood, and thus is not an option for virus-naïve donors or when trying to select T cells specific for pathogens that induce a poor memory response.
Clinical results with VSTs
EBV
EBV is a ubiquitous human herpes virus, and over 95% of the adult population is seropositive. After primary infection, the virus persists in latent form in B lymphocytes and epithelial cells in the nasopharynx, and periodic replicative reactivations in B cells are tightly controlled by a strong viral antigen-specific T-cell response such that ≥1% to 2% of circulating T cells in a normal EBV seropositive individual may be specific for EBV.13 After HSCT, EBV-infected B cells that would normally be controlled by an effective EBV-specific cytotoxic T lymphocyte (CTL) response can outgrow, resulting in EBV PTLD. The risk of PTLD is higher in recipients who have received reduced intensity transplant conditioning regimens that include anti-thymocyte globulin or alemtuzumab (Campath), a selectively T-cell–depleted product or cord blood (CB). PTLD developing after HSCT is usually derived from donor B cells, but can be of recipient origin.
EBV is an excellent model for cellular immunotherapy because LCLs can be generated from any donor by infection of peripheral blood mononuclear cells (PBMCs) with the B95-8 laboratory strain, and LCLs are an excellent APC for stimulating EBV-specific T cells from EBV seropositive transplant donors (>95% of cases). Table 2 shows outcomes of patients who received donor-derived EBV-specific T cells generated by stimulation with LCLs.9-12,21,26,27,31,39-47 At St. Jude Children’s Research Hospital and the Baylor College of Medicine, 114 patients received EBV-specific CTLs as prophylaxis or treatment of EBV lymphoma posttransplant. None of the 101 high-risk patients who received CTLs as prophylaxis developed EBV lymphoma compared with 11.5% of controls.31 Moreover, 11 of 13 patients who received EBV-specific CTL as therapy for established EBV lymphoma achieved sustained complete remissions (CRs). Similar response rates with this product have been reported by Memorial Sloan Kettering Cancer Center, which observed complete responses in 10 of 14 patients with EBV lymphoma10 and the Pavia group, who reported responses in patients with rituximab-resistant disease.39
Although response rates have been high in these studies, evaluation of patients who failed to respond has also been informative. In one of the Baylor patients who had an initial response and then progressed, an EBV variant was found with a deletion in the immunodominant epitopes recognized by the infused line.48 In the Sloan Kettering studies, another 3 patients who failed to respond received VSTs that also recognized the LCLs transformed with B-95 but not the strain of EBV expressed by the patients’ tumor.10 In another patient, the donor CTL line was skewed in its EBV response by an HLA antigen A1101, which was present only in the donor, whereas the tumor was derived from recipient cells. The patient subsequently responded to a third-party line that was matched at fewer antigens but had strong EBV activity through a shared HLA antigen.10 This experience illustrates the importance of ascertaining whether the PTLD is derived from donor or recipient cells. More recent studies have used peptides or EBNA-1 protein to stimulate VSTs that were then selected by γ capture (Table 3).22,23,37,38,49-54 Although the numbers are small, the response rates appear similar.
CMV
CMV is a latent β-herpes virus that can result in significant morbidity and mortality in patients post-HSCT.55 Because CMV-specific CD4+ and CD8+ T cells play an important role in immune protection from both primary infection and subsequent reactivations, several groups have investigated whether the adoptive transfer of in vitro expanded donor-derived VSTs can provide protection following HSCT. Investigators in Seattle performed initial studies evaluating this approach,12,56 using fibroblasts infected with the AD169 strain of CMV to stimulate donor T cells, followed by cloning to isolate cytolytic CD8+ CMV-specific T cells for adoptive transfer. In a dose-escalation study, the CMV-specific clones were administered to recipients of matched sibling donor grafts and reconstituted CMV-specific CD8+ T-cell responses without inducing GVHD.12 A number of studies from other centers using a variety of sources of antigen and APCs to generate polyclonal T cells have also shown excellent response rates (Table 2).11,40,57 More recently, both multimer selection and γ capture have also been used with high response rates1,22,38 (Table 3).
ADV
ADV is also a significant cause of morbidity and mortality in HSCT recipients. Many studies have targeted ADV in concert with other viruses (see sections to follow), but the Tubingen group has reported response rates of around 70% using the γ capture strategy to select ADV-specific cells (Table 3).
Multivirus VSTs
Because HSCT recipients who are immunosuppressed often develop multiple viral infections, several groups have explored the use of T cells targeting more than one virus (Table 4).24-26,28,58-64 Our group developed a strategy to generate donor-derived T-cell lines recognizing CMV, EBV, and ADV using an ADV vector expressing the CMV-pp65 protein (Ad5f35-pp65) as a source of antigen, and monocytes and EBV-LCLs as APCs.24 The trivirus-specific T-cell product produced immune reconstitution to CMV and EBV in all recipients and to ADV in patients with a reactivation or infection. Over 90% of patients with active infections or reactivations cleared the virus in association with an increase in VSTs in peripheral blood24 (Table 4). A similar approach was used by Blyth et al in a phase 2 study where 40 allogeneic HSCT recipients received donor-derived CMV and ADV-specific T cells stimulated with the same ADV–CMV-pp65 vector as described above.58 Of note, they compared outcomes with controls that did not receive T cells and saw no significant difference in the rate of CMV reactivation or GVHD, but did observe a significant reduction in the number of reactivations requiring pharmacotherapy and in the duration of such therapy in the VST group.58
In more recent studies, investigators have modified the source of antigen to stimulate donor PBMCs and shown that response rates were similar when using DNA plasmids encoding immunogenic antigens from EBV, CMV, and ADV introduced into APCs by nucleofection25 or using commercially available pepmixes consisting of 15mer peptide libraries derived from viral antigens that overlap by 11 amino acids16 (Table 4). The range of antigens has also been extended to include VZV,59 BK, influenza,65 and HHV6.28 In a study that extended the targeted viruses to five by adding HHV6 and BK, the infused VSTs produced a 94% virological and clinical response rate that was sustained long term.28 The only cause of failure was if the donor was seronegative for the infecting virus.28
Alloreactivity
One initial concern with infusion of VSTs was the potential for alloreactivity either from residual alloreactive T cells in the product or from cross-reactivity between viral and allo-antigens, because in vitro studies have shown that a majority of VST lines possess cross-reactivity against allogeneic HLA molecules.66 However, none of the published studies report an increased incidence of GVHD over what would be expected in the patient population even if the donor was mismatched32 (Tables 2-4). Still, the concern for alloreactivity has meant that most studies have excluded patients with GVHD of ≥grade 2. Moreover, many of these patients are on therapy with steroids, which are a contraindication to VST infusion due to their lytic effect on activated T cells. To address the unmet need in this population (ie, patients who have a high incidence of viral infection), investigators have used gene editing to disrupt the glucocorticoid receptor gene using electroporation of transcription activator-like effector nucleases messenger RNA. Preclinical studies have shown this strategy renders streptamer-selected CMV-specific T cells resistant to glucocorticoids.67 In other preclinical studies, genetic modification has rendered EBV-specific T cells resistant to calcineurin inhibitors.68,69
Third-party VST banks
Despite the high response rates seen with donor-derived VSTs, the need to generate a specific VST product for each patient means that this approach is not feasible for widespread or urgent use, and it is also challenging when the donor is seronegative. One approach to overcome these issues is to develop banks of HLA-matched VSTs from normal seropositive individuals, so the most closely matched line with activity against the infecting virus through a shared antigen can be infused as an immediately available product when needed. There are potential risks with the use of third-party VSTs because most lines will be mismatched at one or more HLA loci, which may result in shorter persistence after transfer thereby limiting the antiviral benefit due to recognition of allo-antigens on the infused line by recipient cells. Conversely, alloreactivity of the line against recipient antigens may increase the risk of GVHD. Although there is one report of bystander-induced liver GVHD after third-party ADV VSTs,70 in general, these concerns have been allayed by the published results (Table 5).10,27,54,64,70-74 Results show that the strategy is feasible and does not induce a higher rate of GVHD than expected while producing significant responses. The first reported study used banked EBV-specific VSTs to treat PTLD after solid organ or HSCT, matching VSTs by low resolution typing and screening for strong killing of donor LCLs in the absence of significant killing of patient-derived phytohemagglutinin blasts.71,75,76 The response rates were 64% and 52% at 5 weeks and 6 months, respectively, and no GVHD was reported.71 In a report from Sloan Kettering, 4 of 5 patients receiving third-party EBV-specific VSTs for PTLD developing after HSCT had complete responses.10,77 Our group used banked third-party multivirus-specific T cells to treat patients with refractory CMV, ADV, or EBV infections in a multicenter study.72 We selected the infused VST line based on both the overall degree of HLA match and also on activity against the infecting virus through a shared HLA allele, which required the analysis of viral epitopes and the HLA-restricting elements in the lines. We observed an overall cumulative incidence of CR/partial response (PR) by day 42 of 74%, with no significant difference between responses observed with each virus.72 The overall response rates for third-party VSTs shown in Table 5 range from 50% to 70%, which is slightly lower than those observed with donor-derived VSTs (Tables 2-4) but nevertheless encouraging. Because the observed persistence is shorter than with donor-derived cells, multiple infusions may be required, and more studies are needed to define attributes of the line associated with response and to determine the mechanism of action of the sustained clinical benefit.
Manufacturing T cells with virus specificity from virus-naïve donors
The highest risk situation for viral reactivation/infection after HSCT is when the donor is virus naïve and the recipient is already infected with the virus. CB is an attractive donor source, especially for the pediatric population, but the T-cell naivety does increase the risk for viral infection. Several groups have shown that VSTs can be primed in vitro from CB,78 and CB-derived T cells targeting CMV, EBV, and ADV have been manufactured using a GMP compliant approach from the 20% fraction of CB units and administered to high-risk patients after CB transplantation.60 This approach used CB donor-derived DCs and LCLs as APCs, either transduced with the Ad5f35CMV-pp65 vector or using viral pepmixes.78 Interestingly, epitope mapping showed that the immunodominant CMV-pp65 epitopes recognized by these T cells differed from T cells manufactured from CMV seropositive adult donors, but in a preliminary report still appeared to confer protection.60 The same strategy is being evaluated clinically in seronegative adult donors.
The above experience and the success of the Berlin patient have stimulated interest in allogeneic-VST therapy for the treatment of HIV. In a recent report, T cells specific for multiple HIV epitopes, irrespective of donor HLA type, were expanded from seronegative adult donors using DCs pulsed with pepmixes selected by a proprietary algorithm. These cells provided broad coverage across all HIV clades, and were stimulated with cytokines for initial priming, followed by re-stimulation with pepmix-pulsed cells. These T cells suppressed viral replication compared with unexpanded CD8+ T cells, and CMV- and EBV-specific T cells derived from the same HIV-seronegative donors. HIV-specific T cells manufactured this way are currently being evaluated in the autologous setting (#NCT02208167) and studies utilizing donor-derived HIV-specific T cells after allogeneic HSCT are planned.79
An alternative approach in the naïve donor is to transduce T cells with a TCR with known viral specificity.80,81 Although this strategy offers a novel, rapid method to generate VSTs from naïve donors, it does impose the additional cost and regulatory requirements of gene transfer. In addition, the strategy is HLA restricted and targeting a single viral epitope increases the risk of viral immune escape. However, a clinical trial is currently underway in the United Kingdom evaluating T cells transduced with a retroviral vector expressing a CMV-specific TCR for high-risk patients after HSCT (Morris et al; http://gtr.rcuk.ac.uk/projects?ref=G0701703).
Future directions and conclusions
In summary, the adoptive transfer of VSTs can restore antiviral immunity and treat viral infections in many patients who fail to respond to conventional therapies after HSCT. HSCT recipients with active disease who receive VSTs in a donor-specific setting have response rates between 70% and 90%, and even in the third-party setting response rates approach 70%. Given that, in the majority of cases, responses are durable with a minimal toxicity profile; this strategy has many advantages compared with pharmacologic therapies, and its broader application is made increasingly possible due to third-party VST banks and rapid manufacture methodologies. Late-phase licensing studies are now in progress evaluating infusion of VSTs post-HSCT, and it will be important to incorporate cost and quality of life analyses in these trials. Future directions include devising strategies that can be used in patients who have a viral infection with active GVHD, and who are receiving steroids and immunosuppressive agents.67-69 Infusions of VSTs genetically modified with chimeric-antigen receptors82 can also be used after strategies to reduce GVHD such as naïve cell depletion,6 TCR α β depletion,83 or CD34 selection,7 with the goal of both preventing GVHD and promoting antiviral and antitumor immunity. In addition, exploring strategies to enhance virus-specific activity in naïve donor-derived T cells will further broaden applicability to some of the highest risk patients after HSCT, and the manufacture of T cells specific for an increasing number of viruses including HIV, respiratory viruses, and human papillomavirus84 as well as fungal antigens, will enhance the acceptance of this novel T-cell therapeutic beyond HSCT.
Acknowledgments
The authors thank Catherine Gillespie for editing and Conrad Russell Cruz for drawing Figure 1.
This work was supported by grants from the National Institutes of Health, National Cancer Institute (PO1 CA94237, P50CA12675, and P01 CA148600) and a Specialized Center of Research Award from the Leukemia Lymphoma Society. H.E.H. is supported by a Dan L. Duncan chair and a Dan L. Duncan Cancer Center support grant (P30CA125123).
Authorship
Contribution: C.M.B. and H.E.H. wrote the manuscript.
Conflict-of-interest disclosure: C.M.B. and H.E.H. have a licensing agreement with Cell Medica. H.E.H. is a founder of ViraCyte, and The Center for Cell and Gene Therapy has a collaborative research agreement with Celgene for genetically modified T cells.
Correspondence: Catherine M. Bollard, Children’s National Health System and The George Washington University, 111 Michigan Ave NW, Washington, DC 20010; e-mail: cbollard@childrensnational.org.