Abstract
Natural killer (NK) cells play an important role in surveillance and elimination of malignant cells. Their spontaneous cytotoxicity was first demonstrated in vitro against leukemia cell lines, and NK cells might play a crucial role in the therapy of leukemia. NK cell activity is controlled by an array of germ line–encoded activating and inhibitory receptors, as well as modulating coreceptors. This biologic feature can be exploited in allogeneic cell therapy, and the recognition of “missing-self” on target cells is crucial for promoting NK cell–mediated graft-versus-leukemia effects. In this regard, NK cells that express an inhibitory killer immunoglobulin-like receptor (iKIR) for which the respective major histocompatibility complex class I ligand is absent on leukemic target cells can exert alloreactivity in vitro and in vivo. Several models regarding potential donor–patient constellations have been described that have demonstrated the clinical benefit of such alloreactivity of the donor-derived NK cell system in patients with adult acute myeloid leukemia and pediatric B-cell precursor acute lymphoblastic leukemia after allogeneic stem cell transplantation. Moreover, adoptive transfer of mature allogeneic NK cells in the nontransplant or transplant setting has been shown to be safe and feasible, whereas its effectivity needs further evaluation. NK cell therapy can be further improved by optimal donor selection based on phenotypic and genotypic properties, by adoptive transfer of NK cells with ex vivo or in vivo cytokine stimulation, by the use of antibodies to induce antibody-dependent cellular cytotoxicity or to block iKIRs, or by transduction of chimeric antigen receptors.
Introduction
Natural killer (NK) cells belong to innate lymphoid immune cells that contribute to antitumor responses without prior sensitization. According to the currently applied innate lymphoid cell nomenclature, NK cells are a prototypical member of the group 1 innate lymphoid cells insofar as they produce interferon γ (IFN-γ) and developmentally require the common cytokine receptor γ chain, the transcriptional repressor inhibitor of DNA binding 2, and T-bet.1 During maturation, NK cells sequentially acquire lineage-specific markers such as CD94, NKp46, CD56, and CD16. Based on the surface density of CD56, the primarily cytokine-producing CD56brightCD16− NK cell subset is distinguishable from the predominantly cytotoxic CD56dimCD16+ subset. Although CD56brightCD16− NK cells constitute a minor fraction of NK cells in the peripheral blood (∼10%), they are enriched in the secondary lymphoid organs and presumably differentiate here to CD56dimCD16+ NK cells. During this maturational process, they upregulate natural cytotoxicity receptors, express perforin, proliferate, and acquire cytolytic activity upon stimulation with the T cell–derived cytokine interleukin 2 (IL-2). In contrast, the majority (∼90%) of the peripheral blood NK cell compartment consists of CD56dimCD16+ NK cells, which display markers of late maturity such as killer immunoglobulin-like receptors (KIRs) that are inhibitory (iKIRs) and CD57, vividly produce perforin to exert effector functions, but display little proliferative capacity upon IL-2 stimulation.2
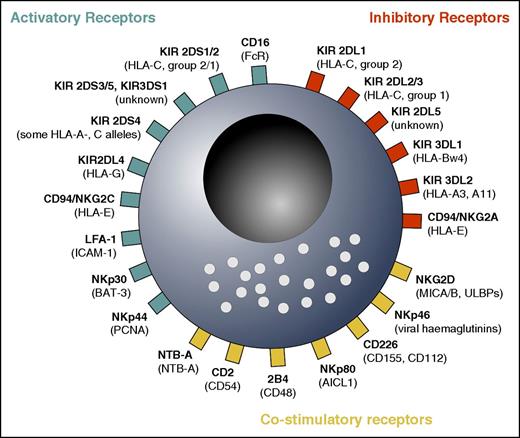
NK cells use different innate receptors to sense their environment and respond to alterations induced by infections or by malignant cell transformation. In a process termed “licensing,” NK cells use iKIRs for “self”–major histocompatibility complex (MHC) class I molecules to maintain a state of responsiveness and to kill target cells that have lost MHC class I.3 In a balanced state, healthy tissue that expresses the self ligands to inhibitory NK cell receptors (ie, certain MHC class I molecules) will be spared from being killed. In contrast, the recognition of missing or downregulated self-MHC class I molecules on tumor cells by licensed NK cells shifts the NK cell receptor balance toward activation. Altogether, the modulation of NK cell activity is controlled by an array of germ line–encoded activating and inhibitory receptors, as well as modulating coreceptors, which are reviewed by Long et al4 and summarized in Figure 1.
Overview over important activatory, inhibitory, and costimulatory NK cell receptors. NK cells integrate various signals in response to leukemic cells. The precise function of some of the receptors is not yet well known, and some activatory receptors might be costimulatory, and vice versa. Note that recent evidence suggests that NKG2D and NKp46 are co-stimulatory receptors because they are not able to induce NK cell activity on their own.4 In addition, KIR2DL4 is, to date, considered to convey activatory rather than inhibitory signals.4 Many other receptors, including cytokine, chemotactic, and adhesion molecule receptors, as well as other costimulatory receptors, are not shown. Ligands are shown in parentheses. AICL; activation-induced C-type lectin; BAT-3, HLA-B-associated transcript 3; CD16, FcRIII receptor; LFA-1, leukocyte functional antigen 1; MICA/B, MHC class I–related chain A/B; NTB, natural killer T- and B-cell antigen; PCNA, proliferating cell nuclear antigen; ULBPs, UL-16 binding proteins.
Overview over important activatory, inhibitory, and costimulatory NK cell receptors. NK cells integrate various signals in response to leukemic cells. The precise function of some of the receptors is not yet well known, and some activatory receptors might be costimulatory, and vice versa. Note that recent evidence suggests that NKG2D and NKp46 are co-stimulatory receptors because they are not able to induce NK cell activity on their own.4 In addition, KIR2DL4 is, to date, considered to convey activatory rather than inhibitory signals.4 Many other receptors, including cytokine, chemotactic, and adhesion molecule receptors, as well as other costimulatory receptors, are not shown. Ligands are shown in parentheses. AICL; activation-induced C-type lectin; BAT-3, HLA-B-associated transcript 3; CD16, FcRIII receptor; LFA-1, leukocyte functional antigen 1; MICA/B, MHC class I–related chain A/B; NTB, natural killer T- and B-cell antigen; PCNA, proliferating cell nuclear antigen; ULBPs, UL-16 binding proteins.
The biological basis for NK cell alloreactivity
Because attempts to exploit autologous NK cells by either in vivo stimulation with IL-25 or the infusion of ex vivo stimulated autologous NK cells6 showed limited clinical efficacy, the focus of research was directed toward the use of NK cells from healthy related or unrelated donors in the context of allogeneic cell therapy.
In this context, donor NK cells that express an iKIR for which the respective ligand is absent on the recipient’s leukemic cells and that concomitantly lack the expression of CD94/NKG2A (because HLA-E molecules are present on all cells and will, as a result, inhibit NK cell functionality) are defined as being alloreactive.7 KIRs are genetically determined and highly polymorphic receptors that recognize allotypic variants of MHC class I alleles (KIR ligands [KIRLs]).8 Molecular cloning has revealed that distinct groups of family members comprise 2 or 3 extracellular immunoglobulin-like domains and, hence, are designated KIR2D and KIR3D, respectively. KIRs with inhibitory activity are long-tailed in their intracytoplasmic region, as indicated by the letter “L” (ie, 2DL1), whereas their activating KIR (aKIR) counterparts are short-tailed, as indicated by the letter “S” (ie, 2DS1). KIR2D family members recognize HLA-C alleles with Lys80 (C2 epitope) or Asn80 (C1 epitope) residues, whereas KIR3D family members recognize HLA-B alleles with a Bw4 supertypic specificity.9 Based on the analysis of the 17 KIR genes and pseudogenes, 2 donor haplotypes are distinguished. KIR A haplotype donors express a canonical gene content, including 6 iKIRs (2DL1, -3, and -4 and 3DL1, -2, and -3) and the activating KIR2DS4, whereas KIR B haplotype donors have a variable gene content and express, in addition to the above-mentioned iKIRs, 1 or more of the B-specific genes: KIR2DS1, -2, -3, -5, KIR2DL2 and/or KIR2DL5.10 The considerable differences that exist in KIR gene content and copy number, together with the extensive allelic polymorphisms, account for the high variability that exists in the genomic KIR region of 2 different individuals.11 Because HLA and KIR segregate independently on different chromosomes and because the expression of KIR genes follows a random stochastic distribution, the repertoire of NK cells with diverging combinations of KIRs is large, and only a minority of HLA-matched donors will also be KIR-KIRL matched.
Models to define NK cell alloreactivity in the context of allogeneic cell therapy
The beneficial effect of a KIR-KIRL mismatched donor–recipient constellation in leukemia has been initially described by the Perugia group.12,13 In pioneering studies, these researchers provided evidence in adult acute myeloid leukemia (AML), but not in adult B-cell precursor (BCP) acute lymphoblastic leukemia (ALL), that transplantation of grafts from KIR-KIRL mismatched donors enhanced survival rates. This graft-versus-leukemia (GVL) effect was attributed to NK cell alloreactivity and predicted by analysis of the HLA types of donor and recipient.12,13 Since then, a number of clinical studies confirmed these observations,14-16 whereas other studies were not able to demonstrate any benefit from selecting a KIR-KIRL mismatched donor when transplanting AML patients.17-21 To explain these potential differences and to dichotomize the relative risk of relapse as a result of a given KIR-KIRL constellation, various models have been proposed. One is the “ligand–ligand” model suggested by the Perugia group, which predicts the relative risk of relapse, considering mismatches between donor and recipient iKIRLs (ie, HLA disparities in graft-versus-host disease [GVHD] direction).12,22 Others failed to demonstrate improved survival when applying this model and performing haploidentical transplants with less-vigorous modes of T-cell depletion.23-25 A second model, the “receptor–ligand” model suggested by the Memphis group,26,27 considers the presence of iKIRs on donor NK cells, together with the absence of the corresponding KIRL, in the recipient’s HLA repertoire. This model, so far, has been the most accurate when performing correlative analyses between the donor KIR phenotype and the risk of relapse in pediatric BCP-ALL patients,26,27 in T cell–depleted matched sibling transplants,28 and in unrelated donor transplants of adult AML patients.29,30 The less commonly applied “gene–gene” model accounts for mismatches on donor and recipient KIR genes31 and has accurately predicted survival rates in KIR haplotype B donors after nonmyeloablative hematopoietic stem cell transplantation (HSCT).32 The Tuebingen group33,34 observed that patients homozygous for a HLA-C1 alleles have a poorer outcome than other patients. As a result of these conceptual differences, the term “KIR-KIRL mismatch” is used inconsistently, and this inconsistency may explain, in part, why some of the models are more or less able to predict outcome. In Figure 2, possible constellations of alloreactivity are shown.
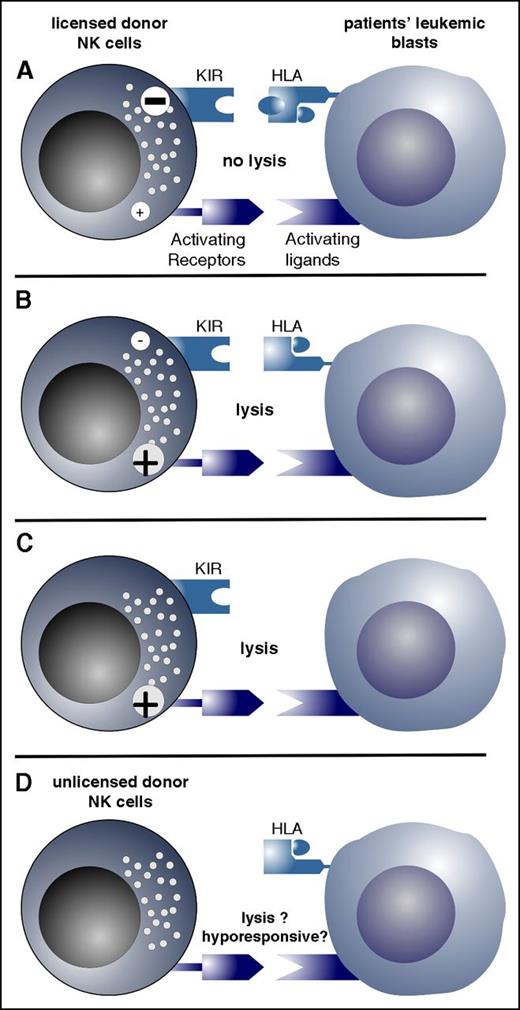
Possible constellations of NK cell alloreactivity are depicted. (A) Licensed donor NK cells (ie, NK cells that have iKIRs for self–HLA class I) are inhibited via engagement of the iKIR by the recipients’ ligands (HLA class I), which exert a strong inhibitory signal; thus, these donor NK cells cannot lyse recipients’ leukemic blasts (NK cell nonalloreactivity). (B) iKIRs of licensed NK cells are not engaged by the KIRLs, and the donor NK cells are activated and lyse recipients’ leukemic blasts (NK cell alloreactivity). (C) Recipients’ blasts lack HLA class I expression and, therefore, cannot inhibit donor NK cells, resulting in activation of donor NK cells. (D) NK cells do not express KIRs and, therefore, have not been licensed. KIR-negative NK cells are one of the earliest lymphocyte populations that reconstitute after T cell–depleted HSCT. These NK cells are hyporesponsive but might become responsive upon cytokine stimulation.
Possible constellations of NK cell alloreactivity are depicted. (A) Licensed donor NK cells (ie, NK cells that have iKIRs for self–HLA class I) are inhibited via engagement of the iKIR by the recipients’ ligands (HLA class I), which exert a strong inhibitory signal; thus, these donor NK cells cannot lyse recipients’ leukemic blasts (NK cell nonalloreactivity). (B) iKIRs of licensed NK cells are not engaged by the KIRLs, and the donor NK cells are activated and lyse recipients’ leukemic blasts (NK cell alloreactivity). (C) Recipients’ blasts lack HLA class I expression and, therefore, cannot inhibit donor NK cells, resulting in activation of donor NK cells. (D) NK cells do not express KIRs and, therefore, have not been licensed. KIR-negative NK cells are one of the earliest lymphocyte populations that reconstitute after T cell–depleted HSCT. These NK cells are hyporesponsive but might become responsive upon cytokine stimulation.
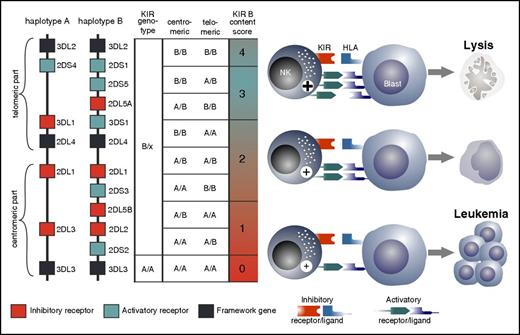
Recently emerging evidence indicates that not only the presence of iKIRs but also the presence of the activating KIR2DS1 regulates NK cell cytotoxicity against lymphoblastoid cell lines,35 adult AML and BCP-ALL,36-39 and pediatric AML and BCP-ALL.40,41 The inclusion of KIR haplotype variability has added an additional layer of complexity to the question of which donor will be the “optimal” donor. Elegant studies in adult AML patients demonstrated that the selection of an HLA-matched unrelated donor who possesses aKIRs next to iKIRs will confer a significantly higher event-free survival.36-38 Depending on their exact position on the KIR locus, distinct centromeric (Cen) and telomeric (Tel) gene-content motifs are described in KIR haplotype A and B donors. In a large cohort study, Cen- and Tel-B motifs both contributed to relapse protection, but Cen-B homozygosity had the strongest independent protective effect.37 This was particularly true for recipients with 1 or 2 C1-bearing HLA-C allotypes.42 In an attempt to calculate the relative risk of relapse after transplant, the so-called KIR B content score can be calculated (http://www.ebi.ac.uk/ipd/kir/donor_b_content.html) by scoring the number of Cen-B and/or Tel-B motifs in each genotype, thus allowing the grouping of donors into the categories of neutral, better, and best. In a large adult cohort study, matched unrelated donors with a KIR B content score of 2 or more conferred a distinctly higher event-free survival in patients with AML, but not ALL.37 A similar result was obtained in children with BCP-ALL after haploidentical T cell–depleted transplantation: not only the presence of KIR haplotype B but, even more so, the selection of donors with a high KIR B content score >2 conferred better protection against relapse.43 In conclusion, a number of association studies suggest that a donor with an iKIR-KIRL mismatch toward the recipient who possesses aKIRs next to iKIRs and who, ideally, expresses not 1, but multiple, aKIRs will be a potentially optimal donor that may promote clinically relevant NK cell alloreactivity (Figure 3). However, given the complexity of the transplant procedure and the strong linkage disequilibrium of selected aKIR genes to other KIR genes, further clinical studies incorporating functional NK cell analysis are needed to conclusively answer the question of whether aKIRs directly contribute to NK cell alloreactivity.
Defining the optimal donor. Based on the KIR haplotype A that has a fixed gene content of 6 iKIRs and 1 aKIR, and the haplotype B that has a highly variable gene content and up to 5 aKIRs, donors can be assigned either to KIR genotype A/A (ie, homozygous for A haplotypes) or to B/x (having 1 or 2 B haplotypes). The Cen and Tel regions can reassemble to form recombinant haplotypes. Genetic association studies suggest that an optimal alloreactive donor will be one with an iKIR-KIRL mismatch and who possesses multiple aKIRs (haplotype B) next to iKIRs. The KIR B content score mainly reflects the number of aKIRs, and the highest score is associated with the highest number of aKIRs. NK cells from such donors with a high KIR B content score should, in principle, exert strong alloreactive antileukemic NK cell activity, and it has been shown that transplantation with donors homozygous for Cen KIR B haplotypes is associated with the lowest level of relapse and highest overall survival.36,42,43 The KIR B content score can easily be determined by KIR genotyping. Note that the majority of haplotype B donors do not express all genes shown in the figure.
Defining the optimal donor. Based on the KIR haplotype A that has a fixed gene content of 6 iKIRs and 1 aKIR, and the haplotype B that has a highly variable gene content and up to 5 aKIRs, donors can be assigned either to KIR genotype A/A (ie, homozygous for A haplotypes) or to B/x (having 1 or 2 B haplotypes). The Cen and Tel regions can reassemble to form recombinant haplotypes. Genetic association studies suggest that an optimal alloreactive donor will be one with an iKIR-KIRL mismatch and who possesses multiple aKIRs (haplotype B) next to iKIRs. The KIR B content score mainly reflects the number of aKIRs, and the highest score is associated with the highest number of aKIRs. NK cells from such donors with a high KIR B content score should, in principle, exert strong alloreactive antileukemic NK cell activity, and it has been shown that transplantation with donors homozygous for Cen KIR B haplotypes is associated with the lowest level of relapse and highest overall survival.36,42,43 The KIR B content score can easily be determined by KIR genotyping. Note that the majority of haplotype B donors do not express all genes shown in the figure.
Therapeutic use of allogeneic NK cells
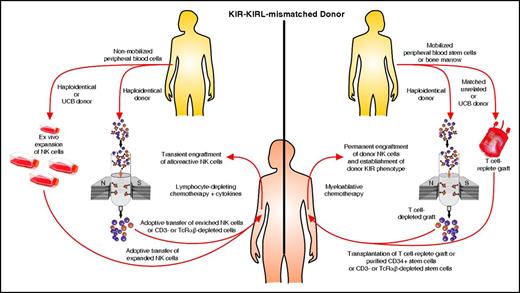
There are two approaches to exploit the antileukemic effect of alloreactive NK cells. One is the adoptive transfer of NK cells from an alloreactive donor, with moderate lympho-depleting chemotherapy to induce homeostatic lymphocyte proliferation with transient expansion of the transferred allogeneic NK cells without the establishment of a permanent donor hematopoiesis. The other is the transplant of hematopoietic stem cells after myeloablative therapy and the permanent establishment of donor hematopoiesis and permanent engraftment of donor NK cells, thus establishing the donor KIR phenotype in the patient. The two approaches are depicted in Figure 4.
Two approaches for exploiting alloreactive NK cells. NK cells from a KIR-KIRL mismatched alloreactive donor or from umbilical cord blood (UCB) can be transferred directly or after ex vivo expansion after mild chemotherapy with transient NK cell proliferation. This will induce only a short-lived antileukemic effect. However, a patient can be permanently engrafted with hematopoietic stem cells from an alloreactive donor or UCB after myeloablative therapy, thus establishing a permanent donor KIR phenotype and antileukemic effect. TcRαβ, αβ T-cell receptor.
Two approaches for exploiting alloreactive NK cells. NK cells from a KIR-KIRL mismatched alloreactive donor or from umbilical cord blood (UCB) can be transferred directly or after ex vivo expansion after mild chemotherapy with transient NK cell proliferation. This will induce only a short-lived antileukemic effect. However, a patient can be permanently engrafted with hematopoietic stem cells from an alloreactive donor or UCB after myeloablative therapy, thus establishing a permanent donor KIR phenotype and antileukemic effect. TcRαβ, αβ T-cell receptor.
Adoptive NK cell therapy
The adoptive transfer of allogeneic NK cells in a nontransplant or transplant setting has been, and is currently being, investigated in clinical trials. In a pioneering clinical study, Miller and coworkers treated extensively lympho-depleted adult AML patients in the nontransplant setting with ex vivo expanded haploidentical NK cells and administered IL-2 in vivo to promote NK cell functionality.44 Remarkably, NK cells persisted and expanded in the subgroup of patients who had been treated with a high-intensity conditioning regimen and who exhibited high intrinsic IL-15 concentrations. In a pilot study involving 10 favorable-prognosis pediatric AML patients, Rubnitz and coworkers were able to show that the combination of low-dose immune suppression with the adoptive transfer of KIR-KIRL mismatched NK cells and in vivo IL-2 treatment after standard chemotherapy was safe and feasible, with long-term survival of all 10 participants.45 Our group recently demonstrated in 8 children with poor prognosis AML, BCP-ALL, and T-ALL that the infusion of ex vivo IL-15-stimulated CD3/CD19-depleted stem cell grafts (containing high numbers of NK cells) was safe and feasible in the haploidentical setting.46
Because NK cells in the nontransplant setting are ultimately rejected, other phase 1 or 2 trials have used ex vivo expanded donor NK cells in patients after more-intensive conditioning. Expansion can be performed using either ex vivo cytokine stimulation or activation via the mbIL15-41BBL-expressing K562 transfectant.47 As sources, both peripheral blood mononuclear cells and umbilical cord blood cells have been used. Although the adoptive transfer of ex vivo expanded NK cells is, to date, considered safe, the clinical benefit has yet to be shown. Given that NK cells, early after transplant, are dysfunctional without the supportive application of IL-2,48 this cytokine was used in combination with adoptive NK cell transfer.44,45,49 However, to achieve in vivo NK cell expansion with IL-2 as the only cytokine, the necessary high doses of IL-2 may induce significant toxicity. Because low-dose IL-2 therapy significantly expands regulatory T-cell numbers and, as such, limits NK cell functionality, an alternative treatment strategy with IL-15 has been conceived. Considering that NK cells crucially depend on IL-15 to achieve optimal differentiation, survival, and effector function,50 the approach to administer IL-15 in vivo is particularly interesting for patients in whom NK cell maturation appears to be blocked in an immature state early after transplant. However, the first-in-human IL-15 study (in malignant melanoma and metastatic renal cell cancer patients) demonstrated significant IL-15 toxicities as a result of elevated proinflammatory cytokine levels,51 and the optimal doses have yet to be defined. Another cytokine-based strategy improves antitumor properties of NK cells using a brief in vitro priming with IL-12, IL-15, and IL-18.52 These so-called cytokine-activated memory-like NK cells are interesting insofar as they exhibit long-lasting increased antitumor properties. In this regard, upregulated IL-2 receptor (CD25) expression renders cytokine-activated memory-like NK cells particularly sensitive to picomolar concentrations of IL-2 and, through this, promotes enhanced IFN-γ production upon re-stimulation with cytokines or tumor cells.53 Interestingly, murine and preclinical in vitro studies suggest that NK cells are actively suppressed by regulatory T cells,54 and it has been assumed that this suppression also occurs early posttransplant. Hence, in a recent study by the Miller group, Bachanova et al55 used adoptive NK cell transfer and in vivo IL-2 administration but concomitantly depleted regulatory T cells with an IL-2 diphtheria-toxin fusion protein.
Allogeneic stem cell transplantation and NK cell reconstitution
To predict whether a theoretically existing KIR-KIRL mismatch will translate into NK cell alloreactivity and, thus, increase GVL effects, it might be useful to integrate the complexity and the kinetics of NK cell reconstitution into the picture. Donor NK cells that reconstitute, develop, and mature in an HLA-disparate recipient will be shaped in the predominantly donor type–like hematopoietic niche56 and are, therefore, donor tolerant and recipient alloreactive, at least in the first months after transplant.26,33,40,57 Keeping the complex process of licensing or “education” of NK cells in mind, it is obvious that the density of donor-derived HLA class I molecules is crucial to the question of whether or not emerging NK cells will be alloreactive. In line with this, it has been postulated that the infusion of megadoses of stem cells will enable better acquisition of NK cell maturity than the infusion of usually applied doses.9 However, many aspects in the processes that are involved in the maturation and education of NK cell populations after HSCT are, at present, unclear. It has been shown that NK cells are among the first lymphocytes to reconstitute to desirable cell numbers (of more than 0.1 × 109/L CD56+ cells) within the first month after transplant, both in adults58,59 and in children,33,40,60 and that the reconstitution kinetics may inversely correlate to relapse probability.59,61 However, the phenotype and cytolytic activity of reconstituting NK cells significantly depends on the preconditioning regime, the source of the graft, and the mode of transplantation. NK cell reconstitution was shown to be impaired in patients who had been exposed to reduced-intensity (nonmyeloablative) conditioning regimens62 (but was enhanced in unrelated cord blood as compared with bone marrow recipients)63,64 and in patients who had received selectively CD3+ or αβ T-cell receptor T cell–depleted grafts that genuinely contain high numbers of donor-derived NK cells with a mature phenotypical and functional profile.33,65 However, despite the presence of acceptable numbers of NK cells, a large number of patients will exhibit a skewing of the NK cell repertoire to the extent that a phenotypically immature subset with little capacity for IFN-γ production (but with some cytotoxic function) will prevail.66 Of interest, individuals with a large pool of NKG2A+KIR− NK cells, few cytotoxic CD3−CD56dim NK cells, and little expression of iKIRs and NKp30 are prone to leukemic relapse.67,68 This would support the observation that KIR-negative unlicensed NK cells early after transplant are hyporesponsive (Figure 2D). In line with the definition that NK cell alloreactivity implies, the presence of iKIRs with varying HLA class I specificities, and the concomitant lack of CD94/NKG2A, analysis of the size and the characteristics of the iKIR NK cell subset is key to the question of which patients experience clinically relevant GVL effects.9,69 Although alloreactive NK cells have been shown to persist and expand in recipients for up to years,40 the size of alloreactive NK cell subsets varies somewhat unpredictably, both in donors and in patients. Phenotypically, the pool of alloreactive NK cells may, in the first place, comprise varying proportions KIR2DL1+, KIR2DL2/3+, KIR3DL1+, and/or KIR2DS1+ subsets. In this regard, KIR2DL1+ NK cells will be inhibited by HLA-C2 recipient’s cells, whereas they will lyse leukemia in C1/C1 recipients. KIR2DL2/3+ NK cells will recognize HLA-C1 but, peculiarly, also low-affinity HLA-C240 ; thus, leukemia of homozygous C2 recipients will only partially be lysed. KIR3DL1+ NK cells have HLA-A- and HLA-B-determined Bw4 supertypic specificity and will, therefore, lyse Bw4− leukemia. And lastly, KIR2DS1+ NK cells will kill leukemia in homozygous HLA-C2 recipients; however, only if the donor is HLA-C1 homozygous. Here, the activating signal of KIR2DS1 will overcome the inhibition of KIR2DL2/3, whereas in HLA-C2 donors, KIR2DS1 will dampen NK cell functionality to prevent autoreactivity.70 A summary of the KIR-KIRL combinations that effectively contribute to NK cell alloreactivity is shown in Table 1.
KIR-KIRL combinations that effectively contribute to NK cell alloreactivity
| KIR-KIRL interaction . | Functional consequences . | NK cell alloreactivity toward . |
|---|---|---|
| KIR2DL1–HLA-C2 | Inhibition in HLA-C2+ patients | Lysis in HLA-C1+ patients |
| KIR2DL2/3–HLA-C1 | inhibition in HLA-C1+ patients | Partial lysis in HLA-C2+ patients* |
| KIR3DL1–HLA-Bw4 | Inhibition in patients with Bw4+ supertypic specificity | Lysis in Bw4− patients |
| KIR2DS1–HLA-C2 | Activation in HLA-C2+ patients | Lysis in HLA-C2+ patients (only when donor is HLA-C1/C1) |
| KIR-KIRL interaction . | Functional consequences . | NK cell alloreactivity toward . |
|---|---|---|
| KIR2DL1–HLA-C2 | Inhibition in HLA-C2+ patients | Lysis in HLA-C1+ patients |
| KIR2DL2/3–HLA-C1 | inhibition in HLA-C1+ patients | Partial lysis in HLA-C2+ patients* |
| KIR3DL1–HLA-Bw4 | Inhibition in patients with Bw4+ supertypic specificity | Lysis in Bw4− patients |
| KIR2DS1–HLA-C2 | Activation in HLA-C2+ patients | Lysis in HLA-C2+ patients (only when donor is HLA-C1/C1) |
See Pende et al.40
We have observed that a substantial number of potentially alloreactive donor-derived NK cells expressing only a single iKIR were detectable after HLA-mismatched HSCT. Of interest, KIR2DL2/3 was predominantly expressed, irrespective of the patients’ HLA type. This may explain why patients homozygous for the C1 group (C1/C1) and, therefore, expressing more inhibitory ligands for KIR2DL2/3 had a poorer survival than patients with a C1/C2 or C2/C2 HLA type.33
Next to these well-defined phenotypical properties of human NK cells with inherent potent GVL functionality, additional adaptive NK cell responses have been postulated when a link between posttransplant cytomegalovirus (CMV) infection and relapse protection was documented in AML patients.71,72 It was shown that CMV infection stably imprints the NK cell repertoire,73 thus promoting the clonal expansion of an NKG2C+aKIR+ NK cell compartment.57,74,75 This potentially alloreactive NK cell subset is CD56dim, simultaneously expresses CD57+, and secretes IFN-γ in response to K562 stimulation.76 At the molecular level, the methylation-induced silencing of FcεRγ, SYK, and EAT-2 DNA promoter regions triggers this adaptive CMV-induced conversion of NK cells.76 Functionally, CD56dimFcεRγ− NK cells are prone to secrete cytokines and to exert antibody-dependent cellular cytotoxicity that is not restricted to CMV-infected target cells.76-78 It has been speculated that either MHC class I–like molecules (such as HLA-E whose expression is retained on leukemic cells67 ) trigger NK cell activity directly via NKG2C78 or virally encoded or so-far-unknown non-HLA ligands trigger other activating NK cell receptors71 (such as aKIRs,74 for example). Although the precise mechanisms of adaptive NK cell conversion remain elusive, a simplified view could be that in the context of viral infection, a proinflammatory stimulus disrupts the tumor-induced immunosuppression and promotes NK cell alloreactivity toward leukemia.
Recognition of acute leukemia by alloreactive NK cells
Despite many publications that provide evidence for the relevance of NK cell alloreactivity in the elimination of adult AML, but not BPC-ALL, less knowledge exists as to the significance of NK cell alloreactivity in the context of pediatric leukemia. In line with the data obtained in adult patients, pediatric AML appears to be a target of alloreactive NK cells.26,45,79,80 In contrast to adult BCP-ALL, pediatric BCP-ALL seems to be susceptible to NK cell–mediated target cell lysis.26,27,69,81 The difference in the susceptibility of adult and childhood BCP-ALL to NK cell–mediated lysis has been ascribed, in part, to differing expression of cell adhesion molecules of the β1 (CD29, CD49d) and β2 integrin (leukocyte functional antigen 1) family and the immunoglobulin superfamily (ICAM-1, leukocyte functional antigen 3),22,82 which essentially results in a reduced NK cell–target conjugate formation and activation in the case of adult BCP-ALL. However, with respect to KIR-KIRL incompatibility, it is probably more important that the surface density of MHC class I ligands is higher in pediatric BCP-ALL as compared with AML,79 although it has been demonstrated that the in vitro cytolytic activity of NK cells against BCP-ALL, in part, correlates to the extent of MHC expression.81,83 To allow the prediction of whether a given leukemia will indeed be the target of alloreactive NK cells, the preclinical testing should probably incorporate phenotypical and functional analyses on the clonal NK cell population level,26 as well as functional binding and redirected cytotoxicity assays in the presence of blocking antibodies to well-defined KIR epitopes,40,79 ideally using patient-specific leukemia as target cells. In this regard, the recent generation of antibodies that can discriminate KIR2DS1 from KIR2DL1, KIR3DS1 from KIR3DL1, and KIR2DS2 from KIR2DL3, but not KIR2DL2, has allowed a more precise analysis of potentially alloreactive NK cell subsets that a donor might express toward a given leukemia.84
Alloreactive NK cells promote GVL effects in the absence of GVHD
Why do alloreactive NK cells mediate GVL effects but, obviously, do not promote the induction of or even prevent GVHD12,85 in the context of ex vivo T cell–depleted HSCT? Experimental evidence obtained in mice demonstrates that alloreactive NK cells may lyse donor-derived antigen-presenting cells and T cells,12,22 may produce transforming growth factor β85 and, by this and other means, achieve control over the antigen-driven proliferation of CD4+86 and CD8+ T cells87 in the bone marrow compartment. Of interest, the targeted deletion of recipient dendritic cells or T cells critically depends on a process called trogocytosis, which enables the downstream acquisition of CCR7, a chemokine ligand which is required for the directed lymph node migration of licensed NK cells in response to CCL19 and CCL21.88 This event is negatively regulated by iKIRs and NKG2A but promoted by KIR2DS1.89 Because nonhematopoietic host tissues such as the skin, which is commonly affected by T cell–mediated GVHD, lack the expression of activating NK cell receptor ligands, the skin appears to be comparatively resistant to NK cell–mediated attack. It is currently assumed that, in some instances, NK cells might contribute to pathology after GVHD is established.90 In this regard, the mode of transplantation (ie, extent of T-cell depletion) and the mode of NK cell stimulation (ie, adoptive transfer with IL-15/4-1BBL activated and expanded NK cells) may affect GVHD occurrence.91
Overcoming KIR-KIRL-mediated inhibition and increasing the antileukemic effects of NK cells
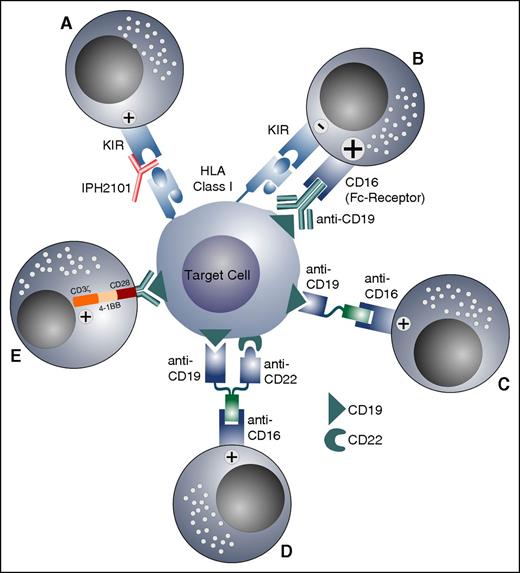
One approach to counteract NK cell suppression induced by the KIR-KIRL interactions is the use of IPH2101, the first-in-class, nondepleting immunoglobulin G4 (IgG4) monoclonal antibody directed against KIR2DL1 and KIR2DL2/3. Indeed, IPH2101 therapy has been effectively able to restore NK cell alloreactivity in adult AML patients,92 and preliminary in vitro experiments also suggest effectiveness toward pediatric BCP-ALL specimens.69 An additional approach to activate alloreactive, but also nonalloreactive, NK cells is the use of monoclonal antibodies directed against antigens expressed on leukemic blasts. It has been shown that the activation of NK cells via the Fc-receptor CD16, which induces antibody-dependent cellular cytotoxicity, provides a strong activatory signal and overrides the inhibitory effects of the KIR-KIRL interaction.93 Because the CD19 antigen is widely expressed in BCP-ALL, an Fc-optimized anti-CD19 antibody was constructed and used posttransplant in selected high-risk patients with pediatric BCP-ALL.94 Other approaches to activate NK cells against BCP-ALL or AML in either the autologous or allogeneic setting, independent of their alloreactive status, might be the future clinical use of the CD16xCD1995 or CD16xCD33 bispecific killer engager96 or the trispecific killer engager CD16xCD19xCD22.97 NK cells and established NK cell lines such as NK-92 can also be redirected toward target antigens expressed on leukemic blasts by chimeric antigen receptors (CARs).98 It has been shown that CAR-NK cells can overcome inhibitory signals and induce specific killing of leukemic cells.99 Although the use of CAR-T cells has, so far, been restricted to autologous T cells, and although CAR-T cells might persist very long, resulting in on-target/off-tumor effects, CAR-NK cells could be adoptively and transiently transferred from allogeneic donors, or even off-the-shelf.100 The various approaches to overcome the KIR-KIRL-mediated inhibition and to increase the cytotoxicity toward leukemic cells in the autologous or allogeneic setting are shown in Figure 5.
Strategies to overcome the KIR-KIRL-mediated inhibition of NK cells and to increase their antileukemic response. (A) KIR-KIRL interaction is blocked by a monoclonal antibody, which abolishes the inhibitory signal. (B) KIR-KIRL-mediated inhibition is overridden by the activation of the Fc-receptor (CD16), with an antibody directed against an antigen expressed on leukemic blasts. (C-D) A bispecific killer engager and a trispecific killer engager activate NK cells via the Fc-receptor against antigens expressed on leukemia cells. (E) CAR-NK cells directed against the CD19 antigen are depicted.
Strategies to overcome the KIR-KIRL-mediated inhibition of NK cells and to increase their antileukemic response. (A) KIR-KIRL interaction is blocked by a monoclonal antibody, which abolishes the inhibitory signal. (B) KIR-KIRL-mediated inhibition is overridden by the activation of the Fc-receptor (CD16), with an antibody directed against an antigen expressed on leukemic blasts. (C-D) A bispecific killer engager and a trispecific killer engager activate NK cells via the Fc-receptor against antigens expressed on leukemia cells. (E) CAR-NK cells directed against the CD19 antigen are depicted.
Perspective
Substantial evidence exists that NK cell alloreactivity can be exploited in adult and pediatric patients with AML and probably also in children with BCP-ALL. However, the selection of a donor with a KIR-KIRL mismatch toward a given patient does not per se preclude the subsequent emergence of alloreactive NK cells with high GVL potential. In this regard, additionally expressed aKIRs, the mode of preconditioning, varying kinetics of NK cell reconstitution, prevailing cytokine levels, and presumably still undefined factors all contribute to NK cell alloreactivity. Therapeutic heightening of NK cell–mediated GVL effects can probably be achieved only with a multitiered approach that combines the optimal recognition of leukemia by KIR-KIRL mismatched NK cells, supportive cytokine or monoclonal antibody administration, and abrogation of NK cell–suppressive effects such as iKIR or regulatory T-cell signaling or transforming growth factor β–promoted cytokine signaling. A number of questions remain as to what the absolute number of NK cells is that is needed to achieve a clinical GVL response, how the different and sequentially appearing activating signals are integrated in the NK cell response to leukemia, what the direct functional evidence is for a role of multiple aKIRs in target cell recognition, and whether NK cells may adapt to leukemia in the sense of acquiring memory-like characteristics.
Acknowledgments
The authors thank Peter-Michael Weber for the graphic art.
This work was supported by the Deutsche Forschungsgemeinschaft grants KFO 183 and SFB 685 (M.C.A., P.L., and R.H.), the “Else Kröner-Fresenius-Stiftung” grant 2012 A296 (M.C.A. and R.H.), the Reinhold-Beitlich-Stiftung (P.L.), the “Stiftung für Krebskranke Kinder Tübingen e.V.,” the Jose-Carreras Leukemia Foundation, and the “Stefan-Morsch-Stiftung” (R.H.).
Authorship
Contribution: M.C.A. and R.H. wrote the manuscript; and P.L. contributed data and critically reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rupert Handgretinger, Department of Pediatric Hematology and Oncology, University Children’s Hospital, Hoppe-Seyler-Str 1, D-72076 Tuebingen, Germany; e-mail: rupert.handgretinger@med.uni-tuebingen.de.