Abstract
Hematologic malignancies provide a suitable testing environment for cell-based immunotherapies, which were pioneered by the development of allogeneic hematopoietic stem cell transplant. All types of cell-based therapies, from donor lymphocyte infusion to dendritic cell vaccines, and adoptive transfer of tumor-specific cytotoxic T cells and natural killer cells, have been clinically translated for hematologic malignancies. The recent success of chimeric antigen receptor–modified T lymphocytes in B-cell malignancies has stimulated the development of this approach toward other hematologic tumors. Similarly, the remarkable activity of checkpoint inhibitors as single agents has created enthusiasm for potential combinations with other cell-based immune therapies. However, tumor cells continuously develop various strategies to evade their immune-mediated elimination. Meanwhile, the recruitment of immunosuppressive cells and the release of inhibitory factors contribute to the development of a tumor microenvironment that hampers the initiation of effective immune responses or blocks the functions of immune effector cells. Understanding how tumor cells escape from immune attack and favor immunosuppression is essential for the improvement of immune cell–based therapies and the development of rational combination approaches.
Introduction
Combinational therapy, including chemotherapy, hematopoietic stem cell transplant (HSCT), small molecules, immunomodulatory drugs, and monoclonal antibodies, can produce long-term remission or cure in different hematologic malignancies. In the continuous effort to develop new therapeutic agents, cellular-based immunotherapies are gaining increasing clinical relevance for hematologic malignancies. The journey of cellular-based immunotherapy stems from the curative effects of allogeneic HSCT, in which the donor’s immune cells significantly contribute to the elimination of host tumor cells in leukemia, lymphoma, and multiple myeloma.1-3 The graft-versus-tumor effect after allogeneic HSCT, however, is frequently associated with the occurrence of graft-versus-host disease, calling for more effective and precise cell-based therapies.
Considering the variety and complexity of cellular interactions and molecular pathways involved not only in promoting effective immune responses, but also in blocking autoreactivity and excessive inflammation, multiple cell-based approaches have been implemented to educate immune responses against tumor cells, while preventing toxicity. Dendritic cell (DC)-based vaccines and adoptive transfer of cell subsets, such as cytotoxic T cells or natural killer cells (NKs), have been used in clinical trials to prevent or treat relapse in both the autologous and allogeneic clinical settings.4-6 More recently, immune cell engineering and, in particular, the adoptive transfer of T cells that express a chimeric antigen receptor (CAR) specific for the CD19 antigen have demonstrated remarkable antileukemia activity.7,8
Because of genomic instability and the effects of cancer immune editing (reviewed elsewhere9,10 ), tumors develop multiple paths to ultimately escape immune recognition and destruction. In this review article, we only describe the tumor-associated escape mechanisms that hamper immune responses in the context of hematologic malignancies. In parallel, we also review how immune cell–based therapies have been developed to overcome immune inhibition and the potential contribution of combinatorial treatment of therapeutic success.
Tumor-associated DC dysfunction
DCs are heterogeneous bone marrow–derived immune cells that play an essential physiological role in the uptake and processing of antigens. Upon antigen processing and exposure to danger/stress signals, such as pathogen-associated molecular patterns, damage-associated molecular patterns (DAMPs), or inflammatory mediators, DCs differentiate into mature cells that express costimulatory molecules (CD80, CD86, or CD40) and secrete chemokines and cytokines critical for priming T- and B-cell responses.11
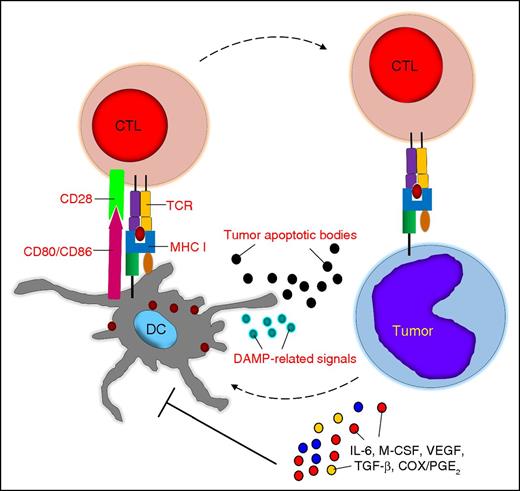
In cancer patients, DCs can engulf altered self-antigens or neoantigens from tumor cells undergoing apoptosis due to hypoxia or nutrient deprivation,12 and in the presence of danger signals, such as DAMP-related signals, they can promote antitumor immune responses.13,14 However, tumor cells and other components of the tumor microenvironment cause quantitative and qualitative defects in the DCs of patients with hematologic malignacies.15-18 Soluble factors such as interleukin-6 (IL-6), macrophage colony-stimulating factor, or vascular endothelial growth factor (VEGF) can block DC differentiation from bone marrow precursors or promote the differentiation of tolerogenic DCs or other immunosuppressive cell subsets.19,20 Tumor-associated factors such as cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2), transforming growth factor-β (TGF-β), and VEGF can also halt DC functions, including phagocytosis, antigen processing, the expression of costimulatory molecules and activation markers, and the secretion of IL-12, which all lead to T-cell tolerance21,22 (Figure 1).
Tumor-associated DC dysfunction. Tumor cells and the tumor microenvironment can cause quantitative and qualitative defects in DCs, which are mostly mediated by soluble factors. CTL, cytotoxic T lymphocyte; M-CSF, macrophage colony-stimulating factor; MHC I, major histocompatibility complex class I; TCR, T-cell receptor.
Tumor-associated DC dysfunction. Tumor cells and the tumor microenvironment can cause quantitative and qualitative defects in DCs, which are mostly mediated by soluble factors. CTL, cytotoxic T lymphocyte; M-CSF, macrophage colony-stimulating factor; MHC I, major histocompatibility complex class I; TCR, T-cell receptor.
Overcoming tumor-associated DC dysfunction
Although dysfunctional in vivo in cancer patients, functionally potent DCs can be generated ex vivo from different sources, including circulating CD14+ monocytes or CD34+ hematopoietic stem cells. Robust evidence showing that DCs can elicit tumor-specific T cells in vitro has powered the clinical translation of DC-based vaccines.23 DCs generated ex vivo and exposed to agents like PGE2, pathogen recognition receptor agonists, and tumor necrosis factor-α (TNF-α) can indeed regain and retain functionality upon inoculation in patients and thus potentially take over dysfunctional resident DCs.24 Several approaches have been used to load ex vivo tumor-associated antigens into DCs. The range of loaded antigens has been broadened by using tumor cell lysates, tumor apoptotic bodies or exosomes, tumor-derived messenger RNA libraries, or tumor-DC fusion; conversely, the range of loaded antigens has been restricted to specific tumor-associated proteins or even epitopes, such as the idiotype portion of B-cell immunoglobulins, NY-ESO-1 or WT1.25-28 Clinical trials using DC-based vaccines have been conducted in patients with lymphoma, myeloma, and leukemia, showing safety, elicitation of immune responses, and promising objective responses in some studies28 but not in others.25,27,29 Although improvement in overall survival granted US Food and Drug Administration approval for a DC-based vaccine for prostate cancer (sipuleucel-T),30 currently, no DC-based vaccine is approved for hematologic malignancies. Although the overall low response rate of the DC-based approach in cancer patients is likely to be multifactorial, one of the most plausible barriers remaining is the eventual blunting of immune responses by the inoculated DCs promoted through the same tumor-associated inhibitory mechanisms that hamper the functions of endogenous DCs. Ex vivo DC engineering by knocking down suppressor of cytokine signaling 131 to enhance antigen presentation, or by inserting an inducible CD40 receptor32 to pharmacologically stimulate the CD40/CD40L pathway, has been developed to increase the in vivo potency of DCs, and some of these approaches have reached clinical translation. The administration of DC-based vaccines in the posttransplant setting offers the advantages of significantly reducing the tumor burden and related immunosuppressive cellular and soluble factors, and of priming T cells that reemerge after cell ablation.33 Finally, combinations of DC-based vaccines with immunomodulatory agents such as lenalidomide or anti-programmed death 1 (PD-1) and anti-programmed death ligand 1 (PD-L1) antibodies are in clinical development and may reinvigorate the field of vaccine strategies for hematologic malignancies34 (Table 1).
Tumor immune evasion strategies
| . | Defects . | Immune cell-based therapies . | Combinations with immune cell–based therapies . |
|---|---|---|---|
| Tumor-associated DC dysfunction | Reduced DC numbers | DCs generated ex vivo | Posttransplant setting |
| Immature or tolerogeneic DCs | Engineered DCs | Immunomodulatory drugs | |
| Treg inhibition | |||
| Checkpoint inhibitors | |||
| Tumor defective antigen presentation and costimulation | Impaired antigen processing and presentation | T cells, NKs, and NKTs expressing CARs | Pharmacologic modulation of the epigenetic profile |
| MHC downregulation and HLA loss | Allogeneic NKs | ||
| Lack of costimulatory molecules | TCR-redirected T cells | ||
| EBV-specific T cells | |||
| Tumor resistance to cytolysis and induction of immune exhaustion | Loss of Fas/TRAIL-R | T cells, NKs, and NKTs expressing CARs | BCL-2 inhibitors |
| Release of soluble death receptors | TCR-redirected T cells | Histone deacetylase inhibitors Proteasome inhibitors | |
| Overexpression of antiapoptotic molecules | EBV-specific T cells | Checkpoint inhibitors | |
| PD-L1 expression | |||
| Tumor-associated immune-suppressive cells | Increased Tregs, TAMs, and MDSCs | DCs or engineered ex vivo expanded | Posttransplant setting |
| T cells, NKs, and NKTs expressing CARs | Lymphodepletion | ||
| Allogeneic NKs | Selective elimination or reprogramming of Tregs, TAMs, and MDSCs | ||
| TCR-redirected T cells | |||
| EBV-specific T cells | |||
| Tumor-associated soluble factors | Immunosuppressive cytokines (IL10, IL6, TGF-β, VEGF) | Additional T-cell engineering with dominant-negative receptors, chemokine receptors, favorable cytokines | Lymphodepletion |
| Chemokines (TARC) | |||
| Tumor-altered immune metabolism | Nutrient deprivation | Additional T-cell engineering to manipulate cell metabolism | Lymphodepletion |
| Hypoxia | IDO inhibitors | ||
| IDO | Adenosine receptor inhibitors |
| . | Defects . | Immune cell-based therapies . | Combinations with immune cell–based therapies . |
|---|---|---|---|
| Tumor-associated DC dysfunction | Reduced DC numbers | DCs generated ex vivo | Posttransplant setting |
| Immature or tolerogeneic DCs | Engineered DCs | Immunomodulatory drugs | |
| Treg inhibition | |||
| Checkpoint inhibitors | |||
| Tumor defective antigen presentation and costimulation | Impaired antigen processing and presentation | T cells, NKs, and NKTs expressing CARs | Pharmacologic modulation of the epigenetic profile |
| MHC downregulation and HLA loss | Allogeneic NKs | ||
| Lack of costimulatory molecules | TCR-redirected T cells | ||
| EBV-specific T cells | |||
| Tumor resistance to cytolysis and induction of immune exhaustion | Loss of Fas/TRAIL-R | T cells, NKs, and NKTs expressing CARs | BCL-2 inhibitors |
| Release of soluble death receptors | TCR-redirected T cells | Histone deacetylase inhibitors Proteasome inhibitors | |
| Overexpression of antiapoptotic molecules | EBV-specific T cells | Checkpoint inhibitors | |
| PD-L1 expression | |||
| Tumor-associated immune-suppressive cells | Increased Tregs, TAMs, and MDSCs | DCs or engineered ex vivo expanded | Posttransplant setting |
| T cells, NKs, and NKTs expressing CARs | Lymphodepletion | ||
| Allogeneic NKs | Selective elimination or reprogramming of Tregs, TAMs, and MDSCs | ||
| TCR-redirected T cells | |||
| EBV-specific T cells | |||
| Tumor-associated soluble factors | Immunosuppressive cytokines (IL10, IL6, TGF-β, VEGF) | Additional T-cell engineering with dominant-negative receptors, chemokine receptors, favorable cytokines | Lymphodepletion |
| Chemokines (TARC) | |||
| Tumor-altered immune metabolism | Nutrient deprivation | Additional T-cell engineering to manipulate cell metabolism | Lymphodepletion |
| Hypoxia | IDO inhibitors | ||
| IDO | Adenosine receptor inhibitors |
Defective tumor antigen presentation and costimulation
When effective antitumor T-cell responses are elicited by functional DCs, the recognition and destruction of malignant cells by effector CD8+ T cells occurs only after peptides derived from tumor-associated antigens are presented in the context of MHC molecules. The downregulation or loss of MHC molecules due to mutations or deletions of HLA loci has been extensively described in hematologic malignancies. Loss of MHC class I expression, 75% of which relates to the aberrant expression of β2-microglobulin, occurs in over 50% of diffuse large B-cell lymphomas.35,36 Similarly, exome sequencing has confirmed that β2-microglobulin is the most frequently mutated gene in Hodgkin lymphoma.37 Loss of MHC class I molecules can cause tumor escape from T cells targeting the NY-ESO-1 antigen, whereas loss of the HLA haplotype can occur in leukemic cells after haploidentical HSCT.38,39 Genes involved in the antigen processing/presentation machinery, such as the transporters associated with antigen processing 1/2, can also be downregulated in lymphomas.40 Finally, mutations, deletions, and rearrangements of the class II MHC transactivator mediate the downregulation of MHC class II molecules, therefore evading CD4+ T-cell recognition.41 In spite of the MHC downregulation and defects in antigen presentation, in patients with Epstein-Barr virus (EBV)-related lymphomas significant clinical responses have been achieved after adoptive transfer of virus-specific cytotoxic T cells.42 In addition, some responses have been obtained in patients with myeloma and acute myeloid leukemia after adoptive transfer of T cells expressing high-affinity TCRs for HLA-A2–restricted NY-ESO-1 or HLA-A2–restricted WT1 peptides.43,44 These data may reflect either the heterogeneity of tumor cell populations or the effect of epigenetic mechanism(s) causing the dynamic regulation of class I expression by tumor cells in vivo when deletions or mutations are not the primary cause of dysfunctional antigen processing.
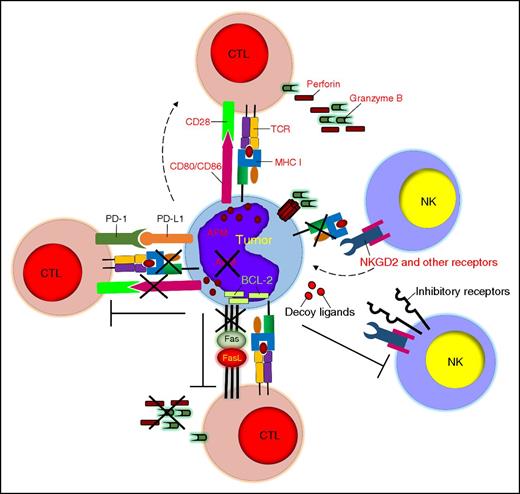
In order to achieve an optimal level of activation, T cells require signaling from costimulatory molecules. One of the most well-characterized costimulatory pathways links the CD28 molecule expressed by T cells to the CD80/86 molecules expressed by antigen-presenting cells. The CD28-CD80/CD86 interaction facilitates the formation of the immunological synapse around the TCR-MHC complex and enhances TCR signaling and T-cell activation.45 TCR binding in the absence of costimulation drives T cells to anergy, a well-described event for hematologic malignancies, which frequently lack the expression of CD80/CD86 molecules46 (Figure 2).
Tumor-defective antigen presentation and costimulation, resistance to cytolysis, and induction of immune exhaustion. Tumor cells can escape immune recognition by downregulating MHC class I molecules and costimulatory molecules and by developing defective antigen processing. Tumor cells can also resist the cytolytic effects of immune cells by overexpressing antiapoptotic molecules or inhibitory ligands. PD-L1 expression by tumor cells causes T-cell exhaustion. APM, antigen-presenting machinery.
Tumor-defective antigen presentation and costimulation, resistance to cytolysis, and induction of immune exhaustion. Tumor cells can escape immune recognition by downregulating MHC class I molecules and costimulatory molecules and by developing defective antigen processing. Tumor cells can also resist the cytolytic effects of immune cells by overexpressing antiapoptotic molecules or inhibitory ligands. PD-L1 expression by tumor cells causes T-cell exhaustion. APM, antigen-presenting machinery.
Prevailing defective antigen presentation and costimulation processes of tumors
T cells genetically modified to express CARs provide a compelling immune cell–based strategy that overcomes both defective antigen presentation and costimulation by tumor cells. CARs are fusion proteins in which a single-chain variable fragment, derived from a monoclonal antibody recognizing a cell surface antigen, is coupled with a signaling molecule (CD3ζ or FcγRI) that activates signaling downstream from the TCR. Similar to antibodies, the antigen recognition of CARs is independent of MHC restriction and overcomes the tumor’s immune ignorance caused by downmodulation of HLA molecules.47 In addition, CARs can be further engineered in tandem to express costimulatory moieties, such as CD28, 4-1BB, or OX40, to promote T-cell costimulation upon engagement of the antigen expressed by tumor cells.48-50 Clinical trials in patients with B-cell lymphoid malignancies receiving CAR-redirected T cells targeting CD19 have now convincingly demonstrated that CAR-mediated T-cell costimulation occurs in vivo51 and promotes over 70% response rate in patients with acute lymphoblastic leukemia.8,52 Encouraging results have also been obtained in patients with chronic lymphocytic leukemia53 and lymphomas.54
Loss or downregulation of HLA class I molecules can be potentially restored by the administration of drugs that modulate the epigenetic profile of tumor cells, including mediators of histone acetylation/deacetylation, histone methylation, and DNA methylation.40 Nevertheless, loss of HLA class I molecules renders tumor cells susceptible to NK-mediated cytotoxicity because of the missing interactions between MHC molecules and inhibitory NK receptors. However, NK dysfunctions are frequently found in hematologic malignancies. NKs isolated from patients with acute myeloid leukemia or multiple myeloma show aberrant increases in inhibitory receptors vs activation receptors, causing inhibition of NK cytotoxic activity.55 Overexpression of CD94/NKG2A inhibitory receptors on NKs is also associated with increased incidence of relapse after allogeneic HSCT.56 Finally, tumor cells can shed MHC class I chain-related genes A and B, which are ligands of the activation receptor NKG2D, to induce chronic stimulation and cell anergy.57 In an effort to revert some of the NK dysfunctions, NKs have been expanded ex vivo and adoptively transferred in patients with leukemia and multiple myeloma. While showing effective cytotoxic activity ex vivo, few objective responses have been reported after infusion of autologous NKs. In sharp contrast, NKs adoptively transferred in the context of allogeneic HSCT appear to persist and promote complete responses in patients with acute myeloid leukemia.58 Engineering of NKs with CARs may further extend the clinical relevance of NK-cell–based immunotherapy by broadening their target spectrum.59
NK T cells (NKTs) are another T-cell subset that has therapeutic potential for hematologic malignancies. NKTs recognize exogenous and endogenous glycolipids presented by the nonclassic MHC-like molecule CD1d.60 NKTs, which are interconnected with DCs, macrophages, CD8+ T cells, and NKs via the release of Th1 and Th2 cytokines, have a recognized role in the host defenses between innate and adaptive immunity. Type I NKTs (also called invariant NKTs) express the invariant TCRα-chain, are readily detectable by α-galactosylceramide–loaded CD1d tetramers, and can target tumor cells of lymphoid or myeloid lineage.61 Increasing interest is directed to the potential for invariant NKTs to be expanded ex vivo and engineered to express CARs, to add other antigen specificities while maintaining their native property of targeting the glycolipid/CD1d.62 Because of their lack of alloreactivity, invariant NKTs also have significant potential in the context of allogeneic HSCT62 (Table 1).
Tumor resistance to cytolysis and induction of immune exhaustion
T cells, NKs, and NKTs induce tumor cell death through perforin-granzyme B–mediated tumor lysis and/or Fas-FasL/TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis. However, tumor cells can either develop intrinsic resistance to immune-mediated lysis or directly promote immune cell exhaustion. Convincing evidence indicates that cancer-initiating cells in hematologic malignancies are inherently resilient to many chemotherapy drugs and irradiation not only because of the expression of drug efflux proteins and relatively quiescent status within the stem cell niche,63 but also because tumor cells can escape immune elimination by developing resistance to perforin-mediated lysis and expressing high levels of the serpin proteinase inhibitor 9 to hijack granzyme B.64 Downregulation of death receptors, overexpression of antiapoptotic proteins (eg, cellular Fas-associated death domain–like IL-1β-converting enzyme–inhibitory protein, inhibitor of apoptosis proteins, and Bcl-2 family proteins) or FasL, and release of decoy death receptors are other well-described means for tumor cells to block or prevent their engagement with effector immune cells.65,66 Constitutive or interferon-γ–induced expression of PD-L1 by tumor cells is emerging as a compelling tumor-associated escape mechanism.67 PD-1–expressing T cells at the tumor site receive suppressive and/or exhaustive signals upon engagement with tumor-expressing PD-L1.68 This effect clearly plays a critical role in the immune escape of hematologic malignancies.69 NKs are also highly sensitive to the PD-1/PD-L1 inhibitory pathway as shown in multiple myeloma.70 Fewer studies are available for NKTs, but preclinical models suggest that these cells are not immune from PD-L1 suppression. Interfering with the PD-1/PD-L1 interaction using PD-1– or PD-L1–blocking antibodies has resulted in remarkable clinical responses as a single treatment in patients with Hodgkin lymphoma, and many studies in leukemia, lymphoma, and multiple myeloma are currently ongoing69 (Figure 2).
Counteracting tumor resistance to cytolysis and induction of immune exhaustion
Although the intrinsic cytolytic properties of effector immune cells cannot realistically be manipulated in vivo or ex vivo, efforts can be envisioned for combining adoptive transfer of tumor-specific T cells or NKs with small molecules targeting antiapoptotic pathways in tumor cells. Recombinant TRAIL and monoclonal antibodies specific for death receptors have been used to activate the extrinsic apoptosis pathway in tumor cells.71 Bcl-2 inhibitors, histone deacetylase inhibitors, and proteasome inhibitors, which upregulate death receptors and proapoptotic proteins in tumor cells, are fueling studies for combination treatment with immune cell–based therapy.72 However, the potential toxicity of these drugs on immune cells must be carefully considered. For example, small molecules interfering with the Bcl-2 pathway may not discriminate between tumor and T cells and cause detrimental effects to the latter because Bcl-2 is involved in the activation and maturation of T lymphocytes after antigen presentation.73 By contrast, the combination of immune cell–based therapies with anti-PD-1– or anti-PD-L1–blocking antibodies is expected to greatly enhance the efficacy of adoptive cell-based therapies. Blocking PD-1/PD-L1 can indeed promote not only the emergence of endogenous T cells that target neoantigens expressed by tumor cells, but can also potentially unleash adoptively transferred immune cells, which would be similarly susceptible to PD-L1–mediated blocking at the tumor site (Table 1).
Tumor-associated immune-suppressive cells
Immune cells in the bone marrow and lymphoid organs are located in specific niches and contribute in regulating normal hematopoiesis. However, stem cell niches also play key roles in the development of malignancies and in maintaining cancer-initiating cells.63 Tumor cells in the bone marrow and lymph nodes are also often surrounded by different types of nonmalignant cells such as regulatory T cells (Tregs), tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs) that create a functionally inhibitory microenvironment, through direct cell-to-cell contact with immune cells or through soluble factors. Although increased frequency of Tregs is widely appreciated in hematologic malignancies,74-77 MDSCs and TAMs only recently became objects of interest on the basis of compelling gene expression profiling data.41,78-82
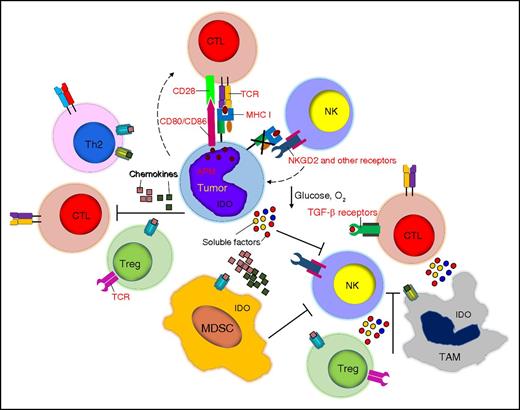
CD4+CD25+Foxp3+ Tregs suppress effector T cells mainly through cell-contact inhibition, secretion of inhibitory cytokines (TGF-β, IL-4, and IL-10) and competition for IL-2 consumption.83,84 NKs are also inhibited in their cytolytic function and expression of activation markers by Tregs via TGF-β.85 TAMs, which are monocytes and macrophages infiltrating the tumor, are frequently defined as M1 and M2 macrophages based on the type of activation signals they receive. Although this categorization is subject to constant discussion and revision, M2 macrophages are generally considered to favor tumor growth, whereas M1 macrophages support immune cell functions through secretion of proinflammatory molecules.86,87 M2 macrophages can directly inhibit T- and NK-cell function and survival by expressing PD-L188 or the nonclassical HLA-G molecule89 and by secreting the inhibitory cytokines IL-10 and TGF-β.90 Through the secretion of CCL22, TAMs further contribute to the recruitment of immunosuppressive Tregs.91 MDSCs consist of a heterogeneous group of cells of myeloid origin, commonly characterized in humans as polymorphonuclear MDSCs (CD11b+CD14−CD15+ or CD11b+CD14−CD66b+) and monocytic MDSCs (CD11b+CD14+HLA-DRlow), although the former are the most abundant in cancer patients.92,93 MDSCs are a major source of T-cell inhibitory cytokines such as IL-10 and TGF-β.92,94 They also induce reactive oxygen species to create oxidative stress and stimulate Tregs, which further exacerbates the immunosuppressive properties of the tumor microenvironment.78,92 MDSCs also directly inhibit NKs via membrane-bound TGF-β95 (Figure 3).
Tumor-associated immune-suppressive cells, soluble factors, and immune cell metabolism. Tumor cells are surrounded by several cell subsets including Tregs, TAMs, and MDSCs, which hamper the function of cytotoxic T cells and NK cells. Chemokines play a critical role in recruiting inhibitory cells, whereas soluble factors such as TGF-β, nutrient deprivation, and hypoxia impair the proliferation and function of effector cells.
Tumor-associated immune-suppressive cells, soluble factors, and immune cell metabolism. Tumor cells are surrounded by several cell subsets including Tregs, TAMs, and MDSCs, which hamper the function of cytotoxic T cells and NK cells. Chemokines play a critical role in recruiting inhibitory cells, whereas soluble factors such as TGF-β, nutrient deprivation, and hypoxia impair the proliferation and function of effector cells.
Overturning tumor-associated immune-suppressive cells
Depletion of inhibitory Tregs, TAMs, and MDSCs is likely critical to enhance the success of vaccine strategies and adoptive transfer of effector cells in hematologic malignancies. In this regard, DC-based vaccines administered after HSCT and infusion of effector cells after myeloid/lymphodepleting regimens are the most simplistic means to achieve this goal. When combined with the adoptive transfer of immune cells, lymphodepletion also reduces the in vivo competition for the homeostatic cytokines IL-7 and IL-15, which are consequently available to the transferred T cells.96 Still, agents capable of modulating the inhibitory cell components of the tumor microenvironment more precisely and with less toxicity are in high demand. The administration of low doses of chemotherapy that cause minimal deleterious effects on T cells and NKs, such as metronomic cyclophosphamide regimens, gemcitabine and trabectedin, can be envisioned to sustain long-term depletion of Tregs, MDSCs, and TAMs.97-99
Selective depletion of Tregs has also been reported using the IL-2–diphtheria toxin conjugate (denileukin diftitox [Ontak]), which triggers Treg apoptosis through irreversible inhibition of protein synthesis, but clinical benefits remain limited due to the high immunogenicity of the molecule.58,100 Several other proteins, including Toll-like receptors, CTLA-4, TNF superfamily receptor OX40, and glucocorticoid-induced TNF receptor directly or indirectly control Treg functions. Based on these discoveries, various agents, such as CpG, Toll-like receptor signaling modulators, and blocking (anti-CTLA-4) or agonistic (anti-OX40 and anti-glucocorticoid-induced TNF receptor) antibodies, have been developed to suppress the activity of Tregs.101-103
TAMs can be targeted by preventing their recruitment/activation at the tumor site or changing their function by taking advantage of their plasticity once they have been recruited within the tumor. Inhibition of the CCL2/CCR2 axis prevents the recruitment of monocytes/macrophages.104 Similarly, macrophages can be inhibited by blocking the CSF1/CSF1R axis or reprogrammed by activating the CD40 pathway.105,106 Finally, as M2 macrophages express CD1d molecules, either constitutively or following exposure to retinoids, they are a potential target for NKTs.107
MDSCs can likewise be selectively inactivated or reprogrammed in vivo. Inhibitors of COX-2, reactive oxygen species, and nitric oxide synthase 2 can block the immunosuppressive functions of MDSCs,108,109 whereas vitamins and derivatives (vitamin A, D3, E, and all-trans retinoic acid) can reprogram MDSCs to nonsuppressive myeloid cells.110,111 Therefore, combinations of strategies that selectively counteract Tregs, TAMs, and MDSCs with DC-based vaccines or adoptive transfer of immune cells are expected to enhance efficacy without significantly increasing toxicity (Table 1).
Tumor-associated soluble factors
Several soluble factors produced by hematologic malignant cells or stromal cells within the tumor environment significantly contribute to impair the survival and functions of effector cells and preserve the immunosuppressive environment. Cytokines such as IL-10, TGF-β, and VEGF are associated with disease progression and poor survival in several hematologic malignancies. Tregs, MDSCs, and M2 macrophages are common sources of IL-10, which causes downregulation of MHC molecules on DCs and tumor cells and directly suppresses Th1 cytokine release by T cells.112 Both soluble and membrane-bound TGF-β can be detected in hematologic malignancies, leading to prolonged inhibition of T-cell proliferation, activation, and cytokine secretion.113 Moreover, both IL-10 and TGF-β contribute to the self-maintenance of the immunosuppressive environment by promoting the generation of induced Tregs.114 Finally, VEGF, in addition to its proangiogenic effects, impairs DC maturation and directly inhibits T-cell proliferation and cytotoxic activity.115 Besides cytokines, chemokines primarily contribute to the recruitment of inhibitory immune cells. For instance, Reed-Sternberg cells are well characterized for their production of CCL22, CCL17/TARC and CCL5/RANTES, which are critical in the recruitment of Tregs, Th2 cells, monocytes, and mast cells116 (Figure 3).
Evading tumor-associated soluble factors
Although the elimination or reprogramming of Tregs, TAMs, and MDSCs per se is anticipated to remove the inhibitory effects of soluble factors produced by these cells, specific countermeasures have been implemented through the direct genetic engineering of ex vivo–expanded immune cells. The inhibitory effects of TGF-β can be blocked by expressing a dominant-negative TGF-β receptor II in tumor-specific T cells, and clinical trials in lymphoma and melanoma patients are ongoing to assess the impact of this modification.117 T-cell costimulation through CARs, IL-15 delivery at the tumor site, and modification of the cytokine/cytokine receptor axis in effector cells can circumvent the inhibition of T-cell proliferation.118-121 Alternatively, the local production of cytokines, such as IL-12, can be helpful in reverting the inhibitory tumor environment, even without directly promoting T-cell proliferation.122,123 Finally, transferred tumor-specific T cells can be engineered to tune chemokine-chemokine receptor pathways and thus favor T-cell recruitment at the tumor site.124 How to prioritize these multiple actions remains largely unknown, and it is anticipated that combinations of multiple factors will prove critical to optimize antitumor effects.125 However, although optimization of effector immune cell trafficking at the tumor site is likely a priority for solid tumors, in hematologic malignancies, engineering T cells to overcome the inhibitory effects of TGF-β could be highly advantageous because TGF-β is almost invariably present in all myeloid and lymphoid tumors (Table 1). In the near future, the development of gene-editing technologies may grant the engineering of tumor-specific T cells to become simultaneously resistant to multiple inhibiting factors.126
Tumor-altered immune cell metabolism
The metabolic activity and alteration in the metabolic program of immune cells, and in particular in T cells and NKs, is gaining significant attention in the field of immune cell-based immunotherapies. The tumor microenvironment in both solid and hematologic malignancies significantly shapes the metabolic program of the immune cells, leading to their dysfunction or death at the tumor site. The transition from naive to effector T cells determines a drastic switch from oxidative phosphorylation to glycolysis as provision to the high demand of protein synthesis for cell growth and effector functions.127 In addition, in rapidly growing tumors, the development of blood vessels is generally overturned by tumor cell outgrowth leading to a limited supply of nutrients. Glucose and amino acids play an important role in T-cell metabolism and the deprivation of these nutrients directly correlates with impaired immune responses. Glucose deprivation causes severe impairment of the proliferation and effector functions of T cells.128 Similarly, tumor cells, MDSCs and stromal cells can sequester cysteine and express the arginase-1 and indoleamine-pyrrole 2,3-dioxygenase (IDO) enzymes, whose main functions are to deprive arginine and tryptophan from the environment, while causing the accumulation of immunosuppressive metabolites that arrest T-cell proliferation and induce apoptosis.129-131 The suboptimal vascularization of the tumor microenvironment, in addition to nutrient deprivation, causes insufficient oxygen supply or hypoxia. Although more prominent in solid tumors than hematologic malignancies, hypoxia impairs T- and NK-cell proliferation, cytolytic activity, the expression of activating receptors and cytokine secretion, which exacerbates the immunosuppression.132 Hypoxic environments also favor the accumulation of adenosine, which in turn can directly inhibit T-cell responses upon binding with the adenosine A2A receptor expressed by activated T cells133 (Figure 3).
Bypassing the tumor-altered immune cell metabolism
Targeting the metabolism or metabolites of the tumor microenvironment may improve the clinical efficacy of cell immune-based therapies. IDO has been implicated in affecting the function of CAR-redirected T cells, and the combination of cell-based immune therapies with IDO inhibitors such as 1-methyl-triptophan and INCB024360 may protect these cells from IDO-mediated inhibition and promote T-cell survival, proliferation, and function.134,135 Developing nontoxic A2A receptor inhibitors, correcting the glucose metabolism of the tumor to reduce its competition with effector immune cells, or reprogramming T-cell metabolism to enhance their function in conditions of glucose deprivation are intriguing new concepts that may have immediate translational application136,137 (Table 1). Finally, a recent study of multiple myeloma also showed that myeloma-infiltrating lymphocytes may be imprinted to adapt to hypoxia, suggesting that a better understanding of the mechanisms of adaptation may be helpful for increasing the functionality of T cells in hypoxia.138
Conclusions and future prospective
Fueled by the unprecedented success of CAR-redirected T cells, cell-based immunotherapy is a realistic and effective approach for the treatment of acute lymphoblastic leukemia. Combinations of cellular therapies with other treatment modalities are likely crucial for curing other hematologic malignancies such as lymphomas, myeloma, and chronic lymphocytic leukemia. However, when considering combination approaches, some degree of prioritization must be taken into account. The current clinical experience suggests that host lymphodepletion before adoptive T-cell therapy is essential for ensuring adequate expansion of infused cells and should be incorporated within clinical protocols. Fludarabine and cyclophosphamide are frequently used to achieve adequate host lymphodepletion.54 However, the development of less toxic but equally effective regiments remains critical. The remarkable clinical activity of checkpoint inhibitors urges their combination with adoptively transferred CAR T cells or TCR-modified T cells or cell-based vaccines. Another clear need is the rapid clinical validation of drugs or antibodies that selectively block or deplete Tregs, MDSCs, and macrophages and their future combination with cell immune-based therapies.
Acknowledgments
The authors thank Debra Taxman for editing the manuscript.
This work was supported in part by a Specialized Center of Research grant from the Leukemia & Lymphoma Society, National Institutes of Health grants RO1 1145564 from the National Heart, Lung, and Blood Institute, RO1CA193130 from the National Cancer Institute, and a Tier 2: Stimulus Award, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill.
Authorship
Contribution: C.S., G.D., and B.S. equally contributed to the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Barbara Savoldo, Department of Pediatrics and Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC 27599; e-mail: bsavoldo@med.unc.edu.