Key Points
Alemtuzumab levels impact acute GVHD, mixed chimerism, and lymphocyte recovery after alemtuzumab, fludarabine, and melphalan RIC HCT.
An in vivo lytic threshold appears to lie near 0.1 to 0.16 μg/mL; targeted dose trials are warranted to optimize outcomes.
Abstract
Reduced intensity conditioning (RIC) allogeneic hematopoietic cell transplantation (HCT) with alemtuzumab, fludarabine, and melphalan is an effective approach for patients with nonmalignant disorders. Mixed chimerism and graft-versus-host-disease (GVHD) remain limitations on success. We hypothesized that higher levels of alemtuzumab at day 0 would result in a low risk of acute GVHD, a higher risk of mixed chimerism, and delayed early lymphocyte recovery and that alemtuzumab level thresholds for increased risks of these outcomes would be definable. We collected data from 105 patients to examine the influence of peritransplant alemtuzumab levels on acute GVHD, mixed chimerism, and lymphocyte recovery. The cumulative incidences of initial grades I-IV, II-IV, and III-IV acute GVHD in patients with alemtuzumab levels ≤0.15 vs ≥0.16 μg/mL were 68% vs 18% (P < .0001), 47% vs 13% (P = .0002), and 32% vs 8%, respectively (P = .005). The cumulative incidence of mixed chimerism in patients with an alemtuzumab level ≤0.15 μg/mL was 21%, vs 42% with levels of 0.16 to 4.35 μg/mL, and 100% with levels >4.35 μg/mL (P = .003). Patients with alemtuzumab levels ≤0.15 or 0.16 to 0.56 μg/mL had higher lymphocyte counts at day +30 and higher T-cell counts at day +100 compared with patients with levels ≥0.57 μg/mL (all P < .05). We conclude that peritransplant alemtuzumab levels impact acute GVHD, mixed chimerism, and lymphocyte recovery following RIC HCT with alemtuzumab, fludarabine, and melphalan. Precision dosing trials are warranted. We recommend a day 0 therapeutic range of 0.2 to 0.4 μg/mL.
Introduction
Reduced intensity conditioning regimens (RIC) containing alemtuzumab, fludarabine, and melphalan have shown promise for the allogeneic hematopoietic cell transplantation (HCT) of pediatric and young adult patients with nonmalignant diseases.1-7 Importantly, survival has been shown to be superior for patients with higher-risk conditions such as hemophagocytic lymphohistiocytosis and combined immune deficiency compared with myeloablative regimens, mostly due to the prevention of early post-HCT regimen-related toxicities such as hepatic veno-occlusive disease and pulmonary hemorrhage.1,2 Late effects following HCT such as gonadal dysfunction may also be decreased following RIC HCT.8
Alemtuzumab-containing RIC HCT regimens are associated with favorably low rates of acute graft-versus-host disease (GVHD),9 especially following high doses of alemtuzumab or proximal timing with regard to graft infusion.5 However, these regimens are also associated with a notable risk of developing mixed donor and recipient chimerism, as high as 70% depending on the timing and dose of alemtuzumab.5,10 Donor contribution to hematopoiesis may remain adequate following RIC HCT, but patients are often treated with additional T-cell or stem cell products following HCT, which necessitate lengthy stays in transplant center locations, and donor lymphocyte infusions carry a notable risk of acute GVHD.5,11 The incidence of graft loss and need for a second allogeneic HCT is estimated at 5%.5 Another downside to RIC HCT with alemtuzumab, fludarabine, and melphalan is the observed delay in immune reconstitution, which is affected by the alemtuzumab dose.12-14 High rates of infectious complications including viral reactivations can be observed.1,2,12,13,15
We previously observed that the dose and schedule of alemtuzumab affects the rates of acute GVHD and mixed chimerism and hypothesized that these effects are due to differences in graft exposure to alemtuzumab.5 Higher doses or more proximal timing (in relation to graft infusion) are expected to result in higher levels of alemtuzumab at day 0 and lead to profound T-cell depletion of the graft, reducing risk of acute GVHD but increasing risk of mixed chimerism. Lower doses or distal dosing schedules would be expected to result in lower levels of alemtuzumab or even clearance by day 0, with increased risk of acute GVHD and decreased risk of mixed chimerism. Other authors have also noted that differences in alemtuzumab dosing alter outcomes following RIC HCT. Alemtuzumab dose reduction studies in adults have shown that increased rates of acute GVHD are observed in patients who are treated with the lowest doses.14 Lower doses of alemtuzumab also result in more robust immune recovery.14,16-18
The pharmacokinetics of alemtuzumab are complicated, and there is tremendous interpatient variability.18,19 Alemtuzumab is a humanized monoclonal antibody targeted against CD52, which is expressed by most lymphocytes. The clearance of alemtuzumab is initially dependent on the availability of CD52 antigen, and early rapid consumption of alemtuzumab results in a short half-life early after administration due to receptor-mediated clearance,19 which is reported by the alemtuzumab package insert to range from 2 to 32 hours following the first 30-mg dose in patients with B-cell chronic lymphocyte leukemia. Following elimination of CD52-expressing targets, the half-life of alemtuzumab lengthens, and the terminal half-life has been reported to range from 1 to 14 days in the current package insert and from 2 to 3 weeks in bone marrow transplant patients.18 A pharmacokinetic–pharmacodynamic model of alemtuzumab was previously developed in patients with B-cell chronic lymphocytic leukemia. Clearance was nonlinear and was affected by the white blood cell count.19
There are limited studies of the pharmacokinetics of alemtuzumab following HCT in pediatric and young adult patients and no reports that define alemtuzumab level thresholds at the time of graft infusion that impact the outcomes of acute GVHD, mixed chimerism, and lymphocyte recovery. Here we present data from 105 patients with nonmalignant diseases to illuminate the significant influence of peritransplant alemtuzumab levels (day 0 ± 72 hours) on the early outcomes of acute GVHD, mixed chimerism, and lymphocyte recovery.
Methods
Patients
Institutional review board approval was obtained for this study. Twenty patients with nonmalignant diseases who were treated with an alemtuzumab, fludarabine, and melphalan RIC were prospectively enrolled on a pharmacokinetic study of alemtuzumab. We also obtained previously collected and cryopreserved plasma samples from an additional 85 patients with nonmalignant diseases who had undergone alemtuzumab, fludarabine, and melphalan RIC HCT. For patients enrolled on the prospective pharmacokinetic study, we used alemtuzumab levels obtained within 8 hours of graft infusion for analyses. For the remaining patients, we used the alemtuzumab level from the stored sample closest to day 0: drawn on day 0 (n = 26), day −1 or +1 (n = 27), day −2 or +2 (n = 15), or day −3 or +3 (n = 17). The terminal half-life of alemtuzumab in bone marrow transplant patients has been reported to be 15 to 21 days,18 and therefore the levels within 72 hours of graft infusion were presumed to be similar to day 0. Indeed, alemtuzumab levels were similar on day −3 and day 0 in patients enrolled in the pharmacokinetic study, with median levels (ranges) of 0.83 (0-2.82) and 0.93 μg/mL (0-2.06 μg/mL), respectively. The median alemtuzumab level on the day of graft infusion was 1.13 μg/mL (range 0-2.91 μg/mL) and was 0.38 μg/mL (range 0-1.77 μg/mL) 2 days following the graft infusion. All patients with a level of 0.1 μg/mL or greater prior to graft infusion still possessed a level of 0.1 μg/mL or greater on day +2.
Demographic and transplant data were collected and are summarized in Table 1. We collected data regarding the development of acute GVHD and mixed chimerism in the first 6 months following HCT. Acute GVHD was graded based on Consensus criteria.20 Mixed chimerism was defined as detection of <95% donor chimerism in >2 consecutive whole blood samples. We collected absolute lymphocyte counts reported by the clinical hematology laboratory at Cincinnati Children’s Hospital from day 0, day +30 (±7 days), and day +100 (±14 days), and lymphocyte subset enumeration results reported by the clinical Diagnostic Immunology Laboratory at Cincinnati Children’s Hospital at day +100 (±45 days). We collected peripheral blood cytomegalovirus (CMV), Epstein-Barr virus (EBV), and adenovirus polymerase chain reaction results reported by the clinical laboratory at Cincinnati Children’s Hospital. Polymerase chain reactions were performed once or twice weekly per standard clinical practice.
Transplant procedures
All patients were treated with an RIC regimen consisting of alemtuzumab, fludarabine, and melphalan as previously described1,21,22 and received a stem cell graft from a bone marrow (n = 101) or peripheral blood stem cell (n = 4) donor. Patients received fludarabine 150 mg/m2 (1 mg/kg in those weighing <10 kg) divided over days −8 to −4 or over days −7 to −3 and melphalan 140 mg/m2 (4.7 mg/kg in those weighing <10 kg) on day −3 or −2. Melphalan doses were decreased by 50% in patients with proven or suspected radiosensitive severe combined immune deficiency or dyskeratosis congenita (n = 6). Patients were classified as having received distal, intermediate, or proximal alemtuzumab schedules as follows. Distal alemtuzumab schedules were given as a dose escalation schedule of 3/10/15/20 mg alemtuzumab over days −22 to −19. Patients weighing <10 kg received 3/10/10/10 mg. Intermediate alemtuzumab schedules were given as 1 mg/kg alemtuzumab divided over days −14 to −10 (0.2 mg/kg/dose). Proximal alemtuzumab schedules were given starting on day −12 or closer to day 0, as either the dose escalation schedule above or as a cumulative 1 mg/kg dose divided over 5 days. Donor, graft source, total nucleated cell dose, and acute GVHD prophylaxis are summarized in Table 1. Patients received routine standard of care antimicrobial prophylaxis, intravenous immunoglobulin replacement, fluid/nutritional support, and other supportive care per routine. Engraftment studies were performed using XY fluorescence in situ hybridization in the case of opposite sex donors and short tandem repeat analysis in the case of same sex donors in the clinical genetics laboratory at Cincinnati Children's Hospital.
Measurement of alemtuzumab levels
Plasma samples were collected from whole blood samples and frozen until measurement. We modified the methodology of Rebello and Hale23 to measure alemtuzumab levels. Methodologies are available on request.
Statistical analyses
Peritransplant alemtuzumab levels, absolute lymphocyte counts, and lymphocyte subset enumerations are presented with descriptive statistics including means, medians, and ranges. The Kruskal-Wallis test was used to compare groups. Tukey boxplots were created in R (R Foundation for Statistical Computing, Vienna, Austria). The Kaplan Meier method was used to generate cumulative incidence curves. Gray’s test was used to compare outcomes of interest including the development of acute GVHD, mixed chimerism, and viral reactivations among patients with differing alemtuzumab level ranges, using the cmprsk package in R. Patients who died prior to the occurrence of an event were censored at the time of death. Classification and regression tree analyses were carried out using the rpart package in R to identify meaningful thresholds of alemtuzumab levels that were associated with increased risks of acute GVHD, mixed chimerism, or higher absolute lymphocyte counts at day +30. The trees were pruned to minimize the cross-validated error. Kaplan Meier survival curves were generated to analyze overall and event-free survival using the survival package in R, and groups were compared with the log-rank test. Event-free survival was defined as survival without retransplantation (death or retransplantation were considered as events). Multivariate analysis of the effect of covariates on the outcomes of acute GVHD, mixed chimerism, and overall and event-free survival were done using Cox hazard regression with the survival package in R. For the analysis of acute GVHD (grades I-IV, II-IV, and III-IV), the initial models included the covariates of alemtuzumab level on the day of graft infusion (continuous variable), donor and patient HLA match, patient age at the time of HCT, and acute GVHD prophylaxis. For the analysis of mixed chimerism, the initial model included the covariates of alemtuzumab level (continuous variable), HLA match, patient age at the time of HCT, GVHD prophylaxis, and underlying diagnosis. Diagnosis was analyzed with several diagnostic categories and also with just 2 groupings of marrow failure vs non–marrow failure, as a diagnosis of marrow failure is known to be associated with a decreased risk of mixed chimerism.5 For the analyses of overall and event-free survival, the initial model included alemtuzumab level, HLA match, patient age at the time of transplant, underlying diagnosis, and occurrence of acute GVHD. Final models were selected using the stepAIC function in the MASS package in R.
Results
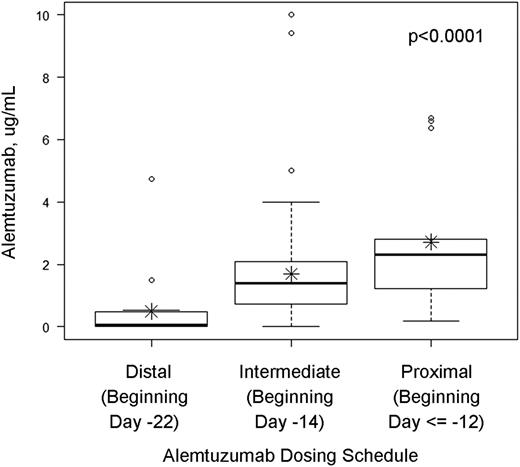
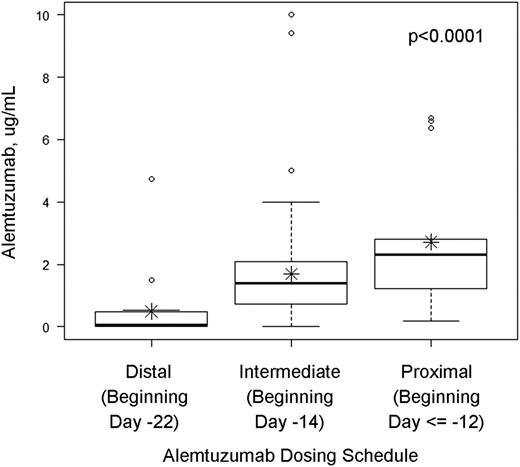
Peritransplant plasma levels of alemtuzumab were measured on the day of graft infusion (±72 hours) in 105 pediatric and young adult patients who received an RIC preparative regimen consisting of alemtuzumab, fludarabine, and melphalan for treatment of a nonmalignant disease. We hypothesized that levels would be highest in patients who received proximal alemtuzumab schedules and lowest in patients who received distal schedules. Indeed, the median levels of alemtuzumab on the day of graft infusion (±72 hours) were 2.3 (range 0.19-6.7), 1.4 (range 0-10.0), and 0.07 μg/mL (range 0-4.73 μg/mL) in the proximal, intermediate, and distal alemtuzumab groups, respectively (P < .0001; Figure 1).
Alemtuzumab levels at the time of graft infusion (±72 hours) in patients treated with distal, intermediate, or proximal alemtuzumab dosing schedules. Median and upper and lower quartiles are given by the dark horizontal lines and boxes. The upper whisker denotes the following. If the 75th percentile plus 1.5 times the interquartile range (IQR) was greater than the largest value in the data set, the upper whisker is the largest value. Otherwise the upper whisker represents the largest value less than the sum of the 75th percentile plus 1.5 times the IQR, and additional outliers were plotted as individual points. For the lower whiskers, if the 25th percentile minus 1.5 times the IQR was less than the smallest value in the data set, the lower whisker represents the smallest value. Otherwise the lower whisker represents the lowest value greater than the 25th percentile minus 1.5 times the IQR, and additional outliers were plotted as individual points. Asterisks represent means.
Alemtuzumab levels at the time of graft infusion (±72 hours) in patients treated with distal, intermediate, or proximal alemtuzumab dosing schedules. Median and upper and lower quartiles are given by the dark horizontal lines and boxes. The upper whisker denotes the following. If the 75th percentile plus 1.5 times the interquartile range (IQR) was greater than the largest value in the data set, the upper whisker is the largest value. Otherwise the upper whisker represents the largest value less than the sum of the 75th percentile plus 1.5 times the IQR, and additional outliers were plotted as individual points. For the lower whiskers, if the 25th percentile minus 1.5 times the IQR was less than the smallest value in the data set, the lower whisker represents the smallest value. Otherwise the lower whisker represents the lowest value greater than the 25th percentile minus 1.5 times the IQR, and additional outliers were plotted as individual points. Asterisks represent means.
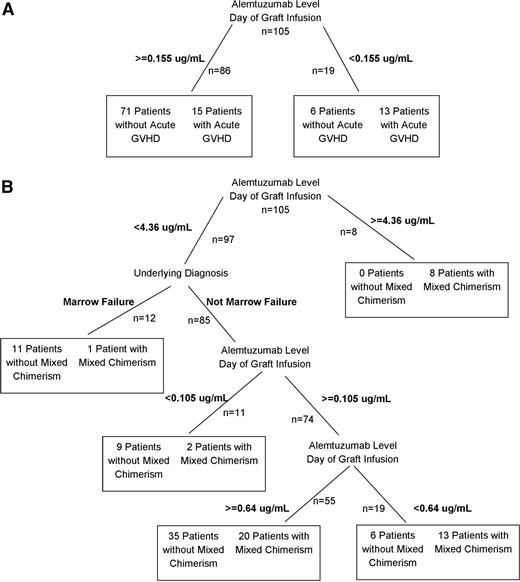
We also hypothesized that the rates of initial acute GVHD (without or prior to the development of mixed chimerism and subsequent interventions) would be highest in patients with absent or nonlytic alemtuzumab levels at the time of graft infusion (due to lack of T-cell depletion of the graft). The lytic level of alemtuzumab in vivo in humans is not clearly known, but we presumed that a level of near 0.1 μg/mL would be lytic in patients based on laboratory studies24 and associated with a decreased rate of acute GVHD. We performed classification tree analysis to determine whether a threshold existed near this value that differentiated patients with high and low risks of acute GVHD, which could be presumed to represent a peripheral blood lytic level. Classification tree analysis suggested that patients with peritransplant (±72 hours) alemtuzumab levels ≤0.155 μg/mL were at high risk to develop acute GVHD (13 of 19 patients), whereas patients with levels >0.155 μg/mL experienced low rates of acute GVHD (15 of 86 patients; Figure 2A). Thus, a level near 0.16 μg/mL may represent a peripheral blood lytic level.
Classification tree analyses. (A) Classification tree analysis of the effect of alemtuzumab level at the time of graft infusion (±72 hours) and HLA match on acute GVHD. (B) Classification tree analysis of the effect of alemtuzumab level at the time of graft infusion (±72 hours) and underlying diagnosis (marrow failure vs non–marrow failure) on the incidence of mixed chimerism.
Classification tree analyses. (A) Classification tree analysis of the effect of alemtuzumab level at the time of graft infusion (±72 hours) and HLA match on acute GVHD. (B) Classification tree analysis of the effect of alemtuzumab level at the time of graft infusion (±72 hours) and underlying diagnosis (marrow failure vs non–marrow failure) on the incidence of mixed chimerism.
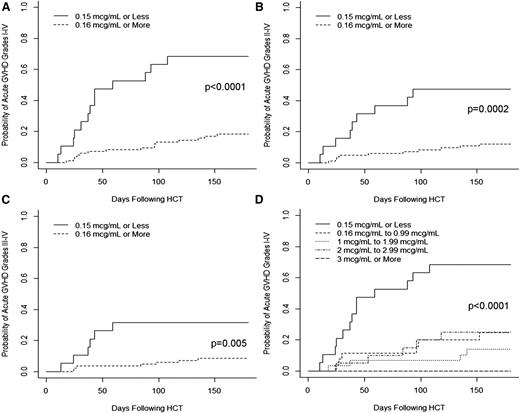
We next examined the cumulative incidences of grades I-IV, II-IV, and III-IV initial acute GVHD following graft infusion (without/prior to development of mixed chimerism and any subsequent interventions) in patients with peritransplant (±72 hours) alemtuzumab levels ≤0.15 vs ≥0.16 μg/mL. The cumulative incidence of grades I-IV acute GVHD before day +180 in patients with alemtuzumab levels of ≤0.15 μg/mL was 68% vs 18% in patients with levels of ≥0.16 μg/mL (P < .0001; Figure 3A). The cumulative incidence of grades II-IV acute GVHD before day +180 in patients with alemtuzumab levels ≤0.15 μg/mL was 47% vs 13% in patients with levels ≥0.16 (P = .0002; Figure 3B). The cumulative incidence of grades III-IV acute GVHD before day +180 in patients with alemtuzumab levels ≤0.15 μg/mL was 32% vs 8% in patients with levels ≥0.16 μg/mL (P = .005; Figure 3C). We also examined the cumulative incidence of acute GVHD in patients based on practical alemtuzumab level stratifications to determine whether increasing levels of alemtuzumab offered additional protection against the development of acute GVHD. As shown in Figure 3D, the risk of acute GVDH grades I-IV was similar among patients with a wide range of alemtuzumab levels from 0.16 to 3 μg/mL, but there was some suggestion that levels ≥3 μg/mL conveyed a minimal risk of acute GVHD, as there were no occurrences of acute GVHD in this group (n = 10).
Impact of peritransplant alemtuzumab levels on the risk of acute GVHD. Cumulative incidence curves of the incidences of (A) grades I-IV acute GVHD, (B) grades II-IV acute GVHD, and (C) grades III-IV acute GVHD stratified by alemtuzumab level of ≤0.15 vs ≥0.16 μg/mL on the day of graft infusion. (D) Cumulative incidence curves of the incidences of grades I-IV acute GVHD stratified by multiple alemtuzumab levels.
Impact of peritransplant alemtuzumab levels on the risk of acute GVHD. Cumulative incidence curves of the incidences of (A) grades I-IV acute GVHD, (B) grades II-IV acute GVHD, and (C) grades III-IV acute GVHD stratified by alemtuzumab level of ≤0.15 vs ≥0.16 μg/mL on the day of graft infusion. (D) Cumulative incidence curves of the incidences of grades I-IV acute GVHD stratified by multiple alemtuzumab levels.
Multivariate analysis was then performed to account for the influence of other factors on the risk of upfront acute GVHD, including patient and donor HLA match, patient age at the time of HCT, and acute GVHD prophylaxis. Higher peritransplant (±72 hours) alemtuzumab levels were associated with decreased risk of acute GVHD grades I-IV (hazard ratio [HR] 0.422, 95% confidence interval [CI] 0.255-0.698, P < .001), grades II-IV (HR 0.477, 95% CI 0.266-0.855, P = .013), and grades III-IV (HR 0.516, 95% CI 0.260-1.025, P = .059; Table 2). Patients who received a graft from a matched unrelated donor were at an increased risk of acute GVHD grades I-IV compared with matched sibling donors (HR 4.300, 95% CI 1.004-18.419, P = .049). A statistically significant increased risk of acute GVHD due to receipt of a mismatched donor graft was not demonstrated in this cohort (HR 2.082, 95% CI 0.404-10.734, P = .381), likely due to small sample size.
We next performed similar analyses to study the effects of alemtuzumab level on the day of graft infusion (±72 hours) on the risk of the development of mixed chimerism. Based on our previous observation of increased rates of mixed chimerism in patients who receive proximal alemtuzumab schedules or higher doses of alemtuzumab,5 we hypothesized that the rates of mixed chimerism would be highest in patients with the highest levels of alemtuzumab, likely due to graft exposure to alemtuzumab and T-cell depletion. Classification tree analysis was carried out to identify meaningful thresholds. We also included underlying diagnosis in the analysis, as a diagnosis of marrow failure has been associated with a decreased risk of mixed chimerism.5 The highest peritransplant (±72 hours) levels of alemtuzumab (≥4.36 μg/mL) were associated with very high rates of mixed chimerism (8 of 8 patients; Figure 2B). The next branch of the classification tree was diagnosis of marrow failure or not, with only 1 of 12 patients with a diagnosis of marrow failure developing mixed chimerism. Following, the tree branched by peritransplant (±72 hours) alemtuzumab levels above and below 0.105 μg/mL, again supporting the conclusion that a lytic threshold exists near 0.1 μg/mL. Only 2 of 11 patients with levels <0.105 μg/mL developed mixed chimerism (18%), whereas 33 of 74 patients with levels >0.105 μg/mL developed mixed chimerism (45%). Further branches did not appear meaningful.
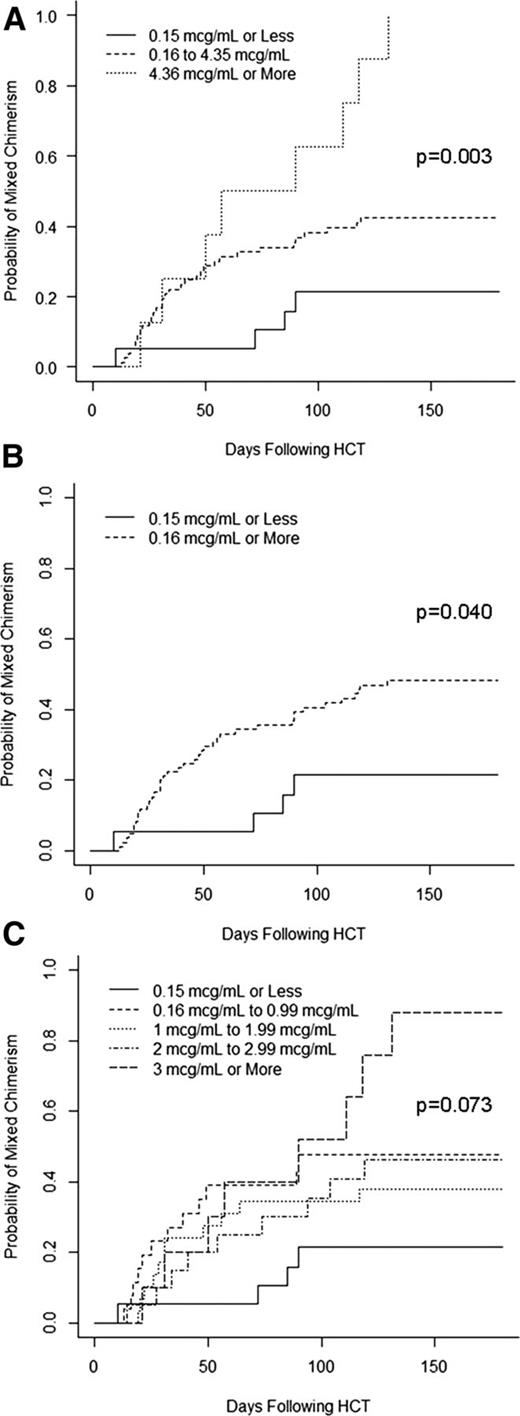
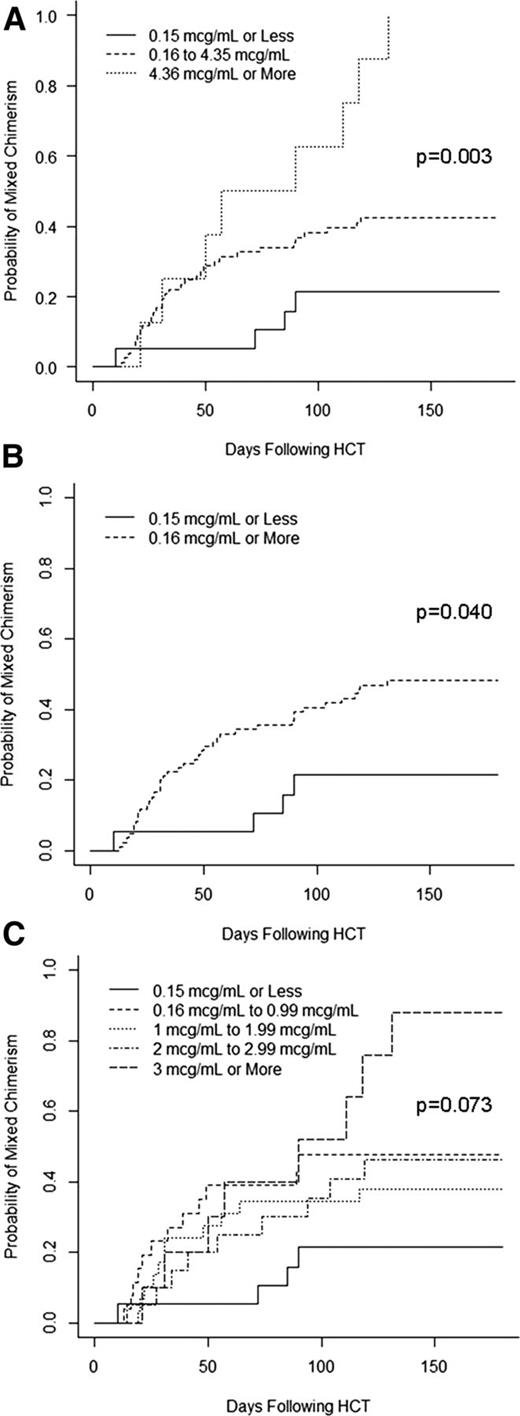
Cumulative incidence curves were then generated to compare the rates of mixed chimerism in patients based on the peritransplant (±72 hours) alemtuzumab level thresholds described above, except that we chose to analyze the lowest threshold group using a level ≤0.15 μg/mL instead of 0.105 μg/mL, so that the results could be more easily considered in relation to the results of the acute GVHD analysis (which used a threshold of ≤0.15 μg/mL). The cumulative incidence of mixed chimerism in patients with an alemtuzumab level ≤0.15 ug/mL on the day of graft infusion (±72 hours) was 21%, whereas the cumulative incidence of mixed chimerism was 42% in patients with levels of 0.16 to 4.35 μg/mL and 100% in patients with levels >4.35 μg/mL (P = .003; Figure 4A). We also analyzed the cumulative incidences of mixed chimerism in patients with alemtuzumab levels simply above or below the presumed lytic threshold of 0.16 μg/mL. The cumulative incidence of mixed chimerism in patients with lytic levels was 48% compared with 21% in patients without lytic levels (P = .040; Figure 4B). Additional practical threshold stratifications were analyzed as in Figure 4C but failed to reveal any escalating risk of mixed chimerism in patients with levels between 0.16 and <3 μg/mL, as the risk of mixed chimerism was similar for all patients within this range (38-48%).
Impact of peritransplant alemtuzumab levels on the risk of mixed chimerism. (A) Cumulative incidence curves of the incidence of mixed chimerism stratified by alemtuzumab levels of ≤0.15, 0.16 to 4.35, and ≥4.36 μg/mL. (B) Cumulative incidence curves of the incidence of mixed chimerism stratified by alemtuzumab levels of ≤0.15 vs ≥0.16 μg/mL. (C) Cumulative incidence curves of the incidence of mixed chimerism stratified by multiple alemtuzumab levels.
Impact of peritransplant alemtuzumab levels on the risk of mixed chimerism. (A) Cumulative incidence curves of the incidence of mixed chimerism stratified by alemtuzumab levels of ≤0.15, 0.16 to 4.35, and ≥4.36 μg/mL. (B) Cumulative incidence curves of the incidence of mixed chimerism stratified by alemtuzumab levels of ≤0.15 vs ≥0.16 μg/mL. (C) Cumulative incidence curves of the incidence of mixed chimerism stratified by multiple alemtuzumab levels.
Multivariate analysis was then performed to account for the influence of other factors on the risk of mixed chimerism, including patient and donor HLA match, patient age at the time of HCT, GVHD prophylaxis, and underlying diagnosis. Increasing levels of alemtuzumab conferred greater risk for the development of mixed chimerism (HR 1.145, 9% CI 1.015-1.292, P = .028), and a diagnosis of marrow failure was associated with a decreased risk of mixed chimerism compared with patients without a diagnosis of marrow failure (HR 0.282, 95% CI 0.068-1.169, P = .081; Table 3).
Of the 44 patients with mixed chimerism, 27 patients were treated with a hematopoietic stem cell boost and/or 1 or more donor lymphocyte infusions. Five patients underwent repeat HCT. The cumulative incidences of receiving a CD34+ selected hematopoietic stem cell boost and/or 1 or more donor lymphocyte infusion(s) and/or a repeat HCT were 15% in patients with a peritransplant (±72 hours) alemtuzumab level ≤0.15 μg/mL and 35% in patients with a peritransplant (±72 hours) alemtuzumab level ≥0.16 μg/mL (P = .066, data not shown).
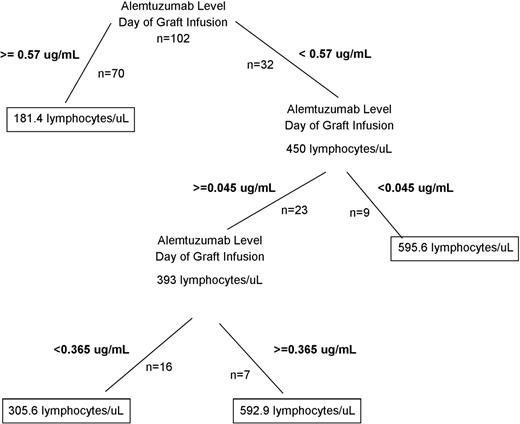
We next determined whether differences in peritransplant (±72 hours) alemtuzumab levels at the time of graft infusion might affect lymphocyte recovery and/or incidence or degree (represented by blood level of viremia) of viral reactivation following HCT. Differences in these outcomes may be due to differences in graft lymphocyte depletion at the time of infusion and/or due to the lingering of alemtuzumab following HCT and continued depletion of developing lymphocytes expressing CD52. The half-life of alemtuzumab has large variability between patients and is influenced by the antigen load of CD52, but the terminal half-life of alemtuzumab in bone marrow transplant patients has been reported to be 15 to 21 days.18,19 Based on a presumed half-life of 15 days, patients with levels of alemtuzumab <0.6 μg/mL would be expected to clear alemtuzumab to <0.15 μg/mL by day +30 following HCT, which may allow more robust lymphocyte recovery in the first months following HCT compared with patients with higher day 0 levels and more prolonged persistence. We therefore first performed regression tree analysis to determine whether thresholds near 0.15 and/or 0.6 μg/mL would be evident with regard to lymphocyte recovery following HCT. Indeed, the first branch of the analysis occurred at a level of 0.57 μg/mL, and the second branch occurred at a level of 0.045 μg/mL (Figure 5). Further branches did not appear meaningful.
Regression tree analysis of the effect of alemtuzumab level on the day of graft infusion (±72 hours) on the absolute lymphocyte count at day +30.
Regression tree analysis of the effect of alemtuzumab level on the day of graft infusion (±72 hours) on the absolute lymphocyte count at day +30.
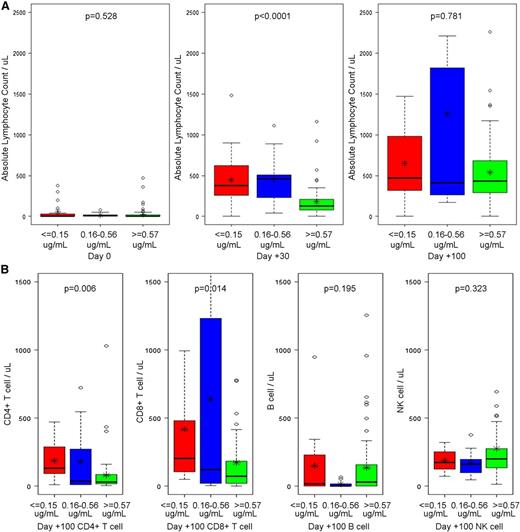
We subsequently examined lymphocyte recovery by the alemtuzumab stratifications suggested by the regression tree analysis above, except that we again chose to use a lowest threshold of ≤0.15 μg/mL to allow for easier consideration of the results in relation to the previous acute GVHD and mixed chimerism analyses. As shown in Figure 6A, we found that patients with a peritransplant (±72 hours) alemtuzumab level ≤0.15 μg/mL and patients with levels from 0.16 to 0.56 μg/mL had higher absolute lymphocyte counts at day +30 (means of 447 and 454 cells/μL) compared with patients with levels ≥0.57 μg/mL (mean of 181 cells/μL; P < .0001). We also observed that early CD4+ and CD8+ T-cell recovery was higher at day +100 in patients with peritransplant (±72 hours) alemtuzumab levels of ≤0.15 and 0.16 to 0.56 μg/mL (Figure 6B). The mean CD4+ T-cell counts in these groups were 188 and 180 cells/μL, respectively, compared with 81 cells/μL in patients with levels ≥0.57 μg/mL (P = .006). The mean CD8+ T-cell counts were 417 and 638 cells/μL, respectively, compared with 175 cells/μL in patients with levels ≥0.57 μg/mL (P = .014).
Impact of peritransplant alemtuzumab levels on lymphocyte and T-cell recovery. (A) Box plots summarizing absolute lymphocyte counts at days 0, +30, and +100 in patients with alemtuzumab levels ≤0.15, 016 to 0.57, or ≥0.57 μg/mL on the day of graft infusion (±72 hours). (B) Box plots summarizing lymphocyte subset enumeration at day +100 in patients with alemtuzumab levels ≤0.15, 016 to 0.57, or ≥0.57 μg/mL on the day of graft infusion (±72 hours). Median and upper and lower quartiles are given by the dark horizontal lines and boxes. The upper whisker denotes the following. If the 75th percentile plus 1.5 times the IQR was greater than the largest value in the data set, the upper whisker is the largest value. Otherwise the upper whisker represents the largest value less than the sum of the 75th percentile plus 1.5 times the IQR, and additional outliers were plotted as individual points. For the lower whiskers, if the 25th percentile minus 1.5 times the IQR was less than the smallest value in the data set, the lower whisker represents the smallest value. Otherwise the lower whisker represents the lowest value greater than the 25th percentile minus 1.5 times the IQR, and additional outliers were plotted as individual points. Asterisks represent means.
Impact of peritransplant alemtuzumab levels on lymphocyte and T-cell recovery. (A) Box plots summarizing absolute lymphocyte counts at days 0, +30, and +100 in patients with alemtuzumab levels ≤0.15, 016 to 0.57, or ≥0.57 μg/mL on the day of graft infusion (±72 hours). (B) Box plots summarizing lymphocyte subset enumeration at day +100 in patients with alemtuzumab levels ≤0.15, 016 to 0.57, or ≥0.57 μg/mL on the day of graft infusion (±72 hours). Median and upper and lower quartiles are given by the dark horizontal lines and boxes. The upper whisker denotes the following. If the 75th percentile plus 1.5 times the IQR was greater than the largest value in the data set, the upper whisker is the largest value. Otherwise the upper whisker represents the largest value less than the sum of the 75th percentile plus 1.5 times the IQR, and additional outliers were plotted as individual points. For the lower whiskers, if the 25th percentile minus 1.5 times the IQR was less than the smallest value in the data set, the lower whisker represents the smallest value. Otherwise the lower whisker represents the lowest value greater than the 25th percentile minus 1.5 times the IQR, and additional outliers were plotted as individual points. Asterisks represent means.
Despite these findings, we did not observe any statistically significant differences in the cumulative incidences of or extent of CMV, EBV, or adenovirus reactivation between patients with lytic or nonlytic level threshold groupings. The cumulative incidences of CMV, EBV, and adenovirus reactivation for patients with peritransplant (±72 hours) alemtuzumab levels of ≤0.15 vs ≥0.16 μg/mL were 37% vs 33% (P = .751), 42% vs 28% (P = .138), and 32% vs 34% (P = .693), respectively.
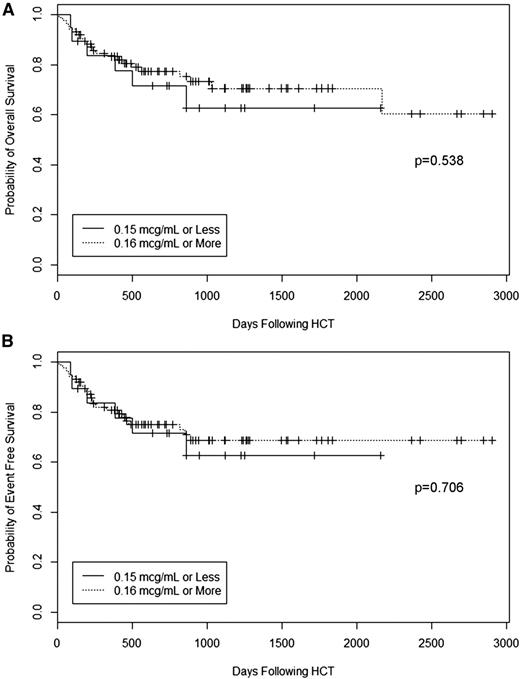
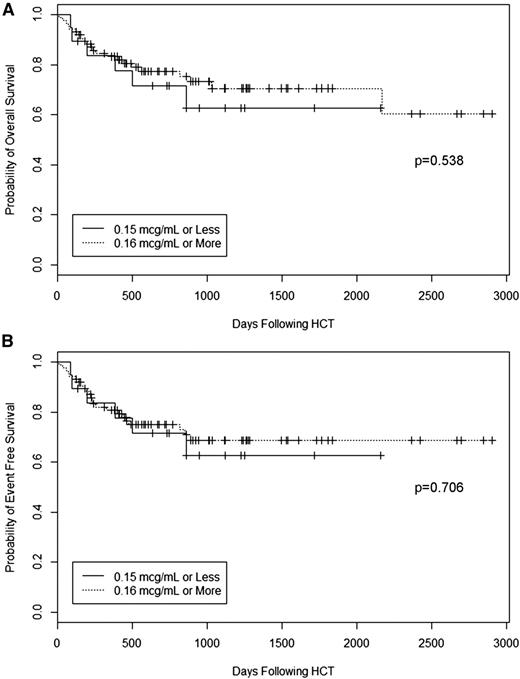
We last performed overall and event-free survival analyses to determine whether peritransplant (±72 hours) alemtuzumab levels affected these outcomes. Event-free survival was defined as survival without retransplantation (death or retransplantation were considered events). We found no effect of alemtuzumab level on overall survival or event-free survival (Figure 7). Five-year overall survival for patients with peritransplant (±72 hours) alemtuzumab levels below the presumed lytic threshold of 0.16 μg/mL was 63%, which was comparable to an overall survival of 71% for patients with higher levels (P = .538). Five-year event-free survival for patients with levels below the presumed lytic threshold of 0.16 μg/mL was 63%, comparable to an event-free survival of 69% for patients with higher levels (P = .706). In multivariate analysis, overall survival and event-free survival remained unaffected by alemtuzumab level. Transplantation with a mismatched donor (HR 5.936, 95% CI 1.285-27.430, P = .023) and the development of grade III-IV acute GVHD (HR 3.112, 95% CI 1.339-7.231, P = .008) were associated with lower overall survival (data not shown). Transplantation with a mismatched donor (HR 5.407, 95% CI 1.180-24.778, P = .030) and the development of grade III-IV acute GVHD (HR 2.942, 95% CI 1.273-6.795, P = .012) were also associated with lower event-free survival (data not shown).
Survival. Kaplan-Meier estimates of (A) overall survival and (B) event-free survival (survival without retransplantation, where death or retransplantation were considered as events) for patients stratified by alemtuzumab level on the day of graft infusion.
Survival. Kaplan-Meier estimates of (A) overall survival and (B) event-free survival (survival without retransplantation, where death or retransplantation were considered as events) for patients stratified by alemtuzumab level on the day of graft infusion.
Discussion
These data represent the first correlation of alemtuzumab level thresholds at the time of transplantation (±72 hours) with the posttransplant outcomes of acute GVHD, mixed chimerism, and lymphocyte recovery in patients with nonmalignant diseases. Our data suggest that an in vivo lytic threshold exists near 0.1 to 0.16 μg/mL and proves that patients with levels of ≤0.15 μg/mL have rates of acute GVHD of >3 times higher than patients who maintain presumed lytic levels (>0.16 μg/mL) at the time of transplantation.
Our data also suggest that, while maintaining a lytic level until the day of graft infusion is beneficial, higher levels do not necessarily confer additional benefit and may be harmful. Very high levels (>4 μg/mL) are associated with high rates of mixed chimerism, and levels >0.57 μg/mL are associated with delayed early lymphocyte and T-cell recovery. We did not observe any effect of peritransplant alemtuzumab levels on day +100 natural killer (NK)-cell recovery, possibly due to the fact that NK cell recovery is brisk, and recovery would be expected to take place within a few weeks of alemtuzumab depletion, which should have occurred prior to day +100 in all patients. Additionally, we observed that a proportion of NK cells may lack expression of CD52 (unpublished observations), which may also allow for NK-cell recovery despite lingering alemtuzumab levels following transplant.
Based on our observations, we believe that targeted dose trials of alemtuzumab are now warranted to determine whether targeted dosing can further optimize and improve patient outcomes. We expect these efforts to be able to minimize the occurrence of initial acute GVHD, decrease rates of mixed chimerism, and encourage early lymphocyte reconstitution. The patients included in our study received various dosing regimens of alemtuzumab, but ongoing detailed pharmacokinetic studies of a uniform 14-day intermediate dosing regimen will result in creation of a pharmacokinetic model to facilitate targeted dosing studies. We would suggest targeting alemtuzumab levels for patients receiving T cell–replete grafts from bone marrow or peripheral blood mononuclear cell graft donors to achieve levels between 0.2 and 0.4 μg/mL on day 0 to achieve a lytic level at the time of graft infusion to protect against the development of acute GVHD, but allow for quick clearance by day +30 after, to optimize lymphocyte recovery. It could alternatively be considered to aim for clearance of alemtuzumab by day 0 in patients with low risk of developing GHVD such as those receiving matched sibling donor grafts. Clearance by day 0 would decrease the risk of mixed chimerism. However, a recent analysis of patients receiving a matched sibling donor transplant using distal alemtuzumab, fludarabine, and melphalan, in which the day 0 levels were presumed to be 0 μg/mL based on a parallel cohort, demonstrated a notable 23% incidence of acute grade II-IV GVHD and a 13% incidence of chronic extensive GVHD.25 This suggests that caution should still be taken when considering how to target alemtuzumab in matched sibling donor transplant patients, as there is no benefit associated with GVHD, and it may still be prudent to aim for lytic levels on day 0.
Our data also point out that mixed chimerism may still occur in patients who lack lytic levels of alemtuzumab on day 0 and suggest that, in addition to optimizing alemtuzumab dosing, additional myelosuppression may benefit RIC regimens containing alemtuzumab, fludarabine, and melphalan. Optimization of melphalan dosing based on patient pharmacokinetics may be useful to achieve a more myeloablative exposure. Alternatively, the inclusion of additional drugs such as thiotepa may be beneficial. Escalation of the CD34+ stem cell dose could also be studied to determine whether a stem cell dose threshold exists that would decrease the risk of mixed chimerism. These and other efforts will continue to improve the outcomes for patients receiving alemtuzumab, fludarabine, and melphalan RIC regimens for treatment of nonmalignant diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for participation in the prospective pharmacokinetic study; all of the care providers at Cincinnati Children's Hospital Medical Center who provided care for patients; and Denise Bellman, Melanie Jordan, Sharon Mitchell, Angie Bonavita, Christine Sper, Linda Carl, Mat Goodridge, Cherie Short, Jessica Gray, and the members of the Molecular Genetics and Diagnostic Immunology Laboratories, especially Lisa Durbin, Kejian Zhang, Jack Bleesing, and Kathryn Quinn.
Authorship
Contribution: R.A.M. designed the research, modified and developed the alemtuzumab assay, collected data, performed statistical analyses, and wrote the manuscript; A.L. supervised the statistical analyses; L.N. contributed to the development of the alemtuzumab assay, measured the alemtuzumab levels, and reviewed the manuscript; and P.A.M., S.J., S.M.D., and A.H.F. contributed patient samples, gave critical advice and support, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rebecca A. Marsh, Division of Bone Marrow Transplantation and Immune Deficiency, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: rebecca.marsh@cchmc.org.