Key Points
Acquisition of the KIT D816V mutation in an early pluripotent progenitor cell confers ISM cases a greater risk for disease progression.
Despite the early acquisition of the KIT mutation, onset of clinical symptoms of ISM is often delayed to middle adulthood.
Abstract
Multilineage involvement of bone marrow (BM) hematopoiesis by the somatic KIT D816V mutation is present in a subset of adult indolent systemic mastocytosis (ISM) patients in association with a poorer prognosis. Here, we investigated the potential involvement of BM mesenchymal stem cells (MSCs) from ISM patients by the KIT D816V mutation and its potential impact on disease progression and outcome. This mutation was investigated in highly purified BM MSCs and other BM cell populations from 83 ISM patients followed for a median of 116 months. KIT D816V–mutated MSCs were detected in 22 of 83 cases. All MSC-mutated patients had multilineage KIT mutation (100% vs 30%, P = .0001) and they more frequently showed involvement of lymphoid plus myeloid BM cells (59% vs 22%; P = .03) and a polyclonal pattern of inactivation of the X-chromosome of KIT-mutated BM mast cells (64% vs 0%; P = .01) vs other multilineage ISM cases. Moreover, presence of KIT-mutated MSCs was associated with more advanced disease features, a greater rate of disease progression (50% vs 17%; P = .04), and a shorter progression-free survival (P ≤ .003). Overall, these results support the notion that ISM patients with mutated MSCs may have acquired the KIT mutation in a common pluripotent progenitor cell, prior to differentiation into MSCs and hematopoietic precursor cells, before the X-chromosome inactivation process occurs. From a clinical point of view, acquisition of the KIT mutation in an earlier BM precursor cell confers a significantly greater risk for disease progression and a poorer outcome.
Introduction
The KIT D816V mutation1 is the most common genetic alteration of systemic mastocytosis (SM),2,3 being present in >95% of adults with indolent SM (ISM) and aggressive SM (ASM).4 Therefore, on its own, this mutation cannot explain the different clinical outcomes5 of indolent cases that show a normal life expectancy (eg, most ISM)6,7 vs the more severe forms of SM, that is, ASM and mast cell leukemia (MCL), in whom patient’s outcome is significantly compromised.8 Thus, secondary genetic lesions on top of the KIT mutation or upon cooperation with a particular genetic background are potentially needed for malignant transformation of ISM into severe disease.9-11
In recent years, we have shown that around one-third of ISM patients carry multilineage myeloid and/or lymphoid involvement of hematopoiesis by the KIT D816V mutation in bone marrow (BM) cell compartments other than mast cells (MCs),4 this being associated with a higher risk for disease progression.7 Altogether, these results suggest that occurrence of the KIT D816V mutation in an early precursor cell could be associated with higher numbers of mutated hematopoietic progenitors, a greater extent of involvement of hematopoiesis by the mutation, and a higher probability of progression of ISM to advanced disease, for example, ASM, MCL, and SM associated to other hematologic non-MC diseases (SM-AHNMD).
The potential occurrence of the KIT mutation in an uncommitted hematopoietic stem and precursor cell (HPC) was first evidenced by the demonstration of this mutation in multiple non-MC myeloid12-15 and also lymphoid12,15,16 cells, in addition to CD34+ HPCs.4 In parallel, involvement of a multipotent CD34+/CD38− HPC has also been demonstrated in other myeloproliferative neoplasms (MPNs), myelodysplastic syndromes (MDSs),17-19 and acute myeloid leukemia.20,21 Moreover, the AHNMD cells from SM-AHNMD patients often carry the KIT D816V mutation,22-24 supporting a common clonal origin for both disease components in an HPC with multilineage potential.
Despite the above, at present it still remains to be established whether, in SM, the somatic KIT D816V mutation occurs at the level of CD34+ HPCs or at an earlier precursor cell. Most likely, early (indolent) forms of SM are accumulative diseases with limited proliferation and expansion of clonal MCs25-27 ; in aggressive cases, additional secondary genetic alterations9 are likely to occur in KIT D816V+ precursors. The earlier the KIT mutation would emerge, the greatest extent of involvement of hematopoiesis would occur with a potentially greater risk for additional secondary genetic alterations and progression of the disease. In this regard, mesenchymal stem cells (MSCs) have been long described as precursor cells that have the ability for differentiation into various mesodermal lineages and tissues.28 MSCs are present in the BM stroma to provide microenvironmental support to HPCs with whom they share an ontogenic link.29,30 Despite MSCs sharing cytogenetic alterations with HPCs in MPN, MDS, and other hematologic disorders,31-34 no study has been reported so far in which the presence of the KIT D816V mutation is investigated in BM MSCs from ISM patients.
Here, we investigated the potential involvement of BM MSCs by the KIT D816V mutation in a cohort of 83 ISM patients in order to determine its potential impact on disease progression and patient outcome.
Methods
Patients
Eighty-three patients (37 men and 46 women; median age at diagnosis, 42 years) diagnosed with ISM at the reference centers of the Spanish Network on Mastocytosis (REMA, Toledo, Spain) were selected from a series of 169 consecutive ISM patients (43 cases having MC-restricted and 40 multilineage KIT mutation according to previously defined criteria7 ) and prospectively included in this study. Patient selection (n = 83) was based on the availability of sufficient (≥3 × 103) highly purified BM MSCs to perform further molecular analyses. Diagnosis and classification of SM was made according to the World Health Organization (WHO) 200835 and the more recent European Competence Network on Mastocytosis36 criteria. Prior to entering the study, patients gave their written informed consent to participate according to the Declaration of Helsinki; the study was approved by the local institutional ethics committees.
Follow-up studies and disease progression
At diagnosis, and subsequently every 6 to 12 months, or whenever disease progression was suspected, a complete clinical and physical examination (including a BM study, a skin biopsy, evaluation of serum baseline tryptase levels and bone lesions) was performed, as previously described.7
Disease progression was defined as transformation of ISM into a more aggressive subtype of mastocytosis,35,36 including smoldering SM (SSM), ASM, and/or SM-AHNMD, after a median follow-up from disease onset of 116 months (range, 28-544 months). In detail, this included emergence of ≥2 “B” findings (ie, organomegaly without impaired organ function; BM infiltration with >30% of focal and dense aggregates of MC; serum tryptase >20 ng/mL; dysplasia or myeloproliferation with non-AHNMD and normal blood counts) in the absence (eg, SSM) or in the presence (ASM) of ≥1 “C” findings (ie, organomegaly with organ failure; BM dysfunction with cytopenia; large osteolytic lesions and/or pathological fractures; malabsorption with weight loss) according to the WHO criteria.35
Immunophenotypic characterization of BM cell populations
Fresh EDTA-anticoagulated BM-aspirated samples collected from the iliac crest were used for multiparameter flow cytometry immunophenotypic analysis of BM cell populations, after they had been stained with a large panel of monoclonal antibodies (MoAbs) (Table 1), following previously described protocols.37
Purification of MCs, MSCs, and other BM cell populations
Isolation of antibody-stained (Table 1) BM cell populations was performed using well-established stain-and-then-lyse-and-wash procedures38 and a 4-way fluorescence-activated cell sorter (FACSAria III; BD Biosciences, San Jose, CA), as described elsewhere.4 For BM cell isolation purposes: MCs were identified as CD117high/CD45+/CD34−/CD3−/CD14−/CD105− cells; monocytes as CD45high/CD14high/CD34−/CD117−/CD3− cells; maturing neutrophils were defined as CD45+/CD34−/CD117−/CD3−/CD14− cells; eosinophils as CD45+/CD13+/CD34−/CD117−/CD3−/CD105−/CD14− sideward scatter (SSC)high with high green and orange autofluorescence cells; CD34+ HPCs were identified as cells with a CD34+/CD45low/CD117−/+/CD13−/+/CD105−/+/CD3−/CD14− phenotype and SSClow/int; and T cells were defined as CD3+/CD45high/CD34−/CD13−/CD14−/CD105−/CD117−/SSClow cells. Identification and purification of MSCs was based on a CD105+/CD13high/CD45−/CD34−/CD14−/CD3− immunophenotype.39 The purity of each of the isolated BM cell populations was systematically >98% in the absence of cross-contamination by MCs (<0.001%) or any other KIT D816V+ BM cell population.
Validation of the CD105/CD13/CD45/CD34/CD14/CD3 antibody combination for the identification of MSCs was performed in each individual sample, by further evaluating their immunophenotypic profile using a broad panel of MoAbs (eg, CD10, CD13, CD34, CD45, CD73, CD90, CD105, CD117, CD146, HLA-DR) including MoAbs for those proteins currently required for the definition of MSCs40,41 (Table 1). In a subgroup of 7 ISM patients, the identity of the isolated CD105+/CD13high/CD45−/CD34−/CD14−/CD3− MSC population was confirmed using an expanded MoAb panel containing additional MSC-associated markers such as the homing cell adhesion glycoprotein (CD44), the platelet-derived growth factor receptor β (CD140b), the nerve growth factor receptor (CD271), the mesenchymal stem cell antigen (MSCA-1), the stage-specific embryonic antigen-4 (SSEA-4), and the stromal cell precursor antigen (STRO-1). Furthermore, the functionality of the isolated CD105+/CD13high/CD45− MSC population was validated in 4 ISM patients carrying both myeloid plus lymphoid multilineage involvement of hematopoiesis by the KIT D816V mutation and mutated MSCs, through in vitro culture and expansion of the isolated MSCs and evaluation of their ability to undergo adipogenic and osteogenic differentiation, following previously described culture and staining conditions.39
DNA extraction and molecular studies
Genomic DNA (gDNA) was extracted from purified cell populations using previously described methods.4 Positivity for the KIT D816V mutation was determined in gDNA of fluorescence-activated cell sorter–purified BM cell populations using a peptide-nucleic acid–mediated polymerase chain reaction (PCR)-clamping technique.4 In order to validate those findings and to rule out any false-positive result due to potential contamination of purified MSCs by other KIT D816V–mutated BM cells, the KIT mutational status of MSCs was also investigated in parallel by an allele-specific oligonucleotide real-time quantitative PCR method42 that provided an accurate measurement of the mutated allele burden of the isolated MSC populations under study. Cases carrying KIT-mutated MSCs systematically showed a mutated allele burden greater than the contamination of the purified MSCs by other BM cells. Multilineage (vs MC-restricted) KIT mutation was defined by the presence of the KIT mutation in maturing/matured BM cell compartments other than MCs (eg, neutrophils, monocytes, eosinophils, and/or lymphocytes, plus the CD34+ HPCs) vs only the MC. MC clonality was further assayed on gDNA from 26 of 46 female patients by the human-androgen receptor (HUMARA) X-chromosome inactivation test.43
Statistical analyses
To assess the statistical significance (set at P < .05) of differences observed between groups, either the Mann-Whitney U or the Pearson χ2 and the Fisher exact tests were used for continuous and categorical variables, respectively (SPSS 20.0, Chicago, IL). Progression-free survival (PFS) curves were estimated according to the Kaplan-Meier method and compared with the Breslow (ie, generalized Wilcoxon) test (SPSS 20.0).
Results
Immunophenotypic and functional characterization of BM MSCs from ISM patients
All purified CD105+/CD13high+/CD45− BM cells showed immunophenotypic features which were fully consistent with previously defined criteria for MSCs39-41 such as: absence of CD11b, CD14, CD19, CD34, and CD45 expression; heterogeneous reactivity for CD10 and HLA-DR; and expression of the CD44, CD73, CD90, CD105, CD140b, CD146, CD271, MSCA-1, SSEA-4, and STRO-1 MSC-associated markers (supplemental Figure 1, available on the Blood Web site).
Ex vivo culture and expansion of purified CD105+/CD13high/CD45− MSCs in 4 ISM cases carrying KIT D816V+ MSCs (median allele burden of 17%; range, 9%-34%) confirmed the presence of the KIT mutation in cultured MSCs from all 4 cases in the first culture passage (median of 17 days of culture; range, 14-26 days) with a median KIT D816V–mutated allele burden of 11% (range, 6%-26%). In contrast, no KIT D816V–mutated MSCs were detected after passage 3 (median of 39 days of culture; range, 27-47 days). After passage 4 (median of 45 days of culture; range, 32-55 days), in 3 of 4 cases, enough cultured cells were obtained to evaluate the adipogenic and osteogenic differentiation of cultured MSCs. Two cases showed normal adipogenic and osteogenic differentiation whereas the third case reached senescence prior to differentiating (supplemental Figure 2).
Presence of the KIT D816V mutation in BM MSCs and its association with ISM disease features at diagnosis
Overall, 22 of 83 ISM patients (27%) had the KIT D816V mutation in purified BM MSCs (Table 2). All KIT D816V–mutated MSC patients showed a mutated allele burden >8% for the purified MSC population (median, 18%; range, 8%-100%). Despite patients who had mutated MSCs having a similar median follow-up and distribution by sex and age at onset, to cases having nonmutated MSCs, both patient groups showed markedly different disease features at diagnosis (Table 2). Thus, patients who had KIT D816V+ MSCs displayed higher levels of BM MC infiltration (median, 0.49% vs 0.08%; P = .008) and greater serum baseline tryptase (median, 170 ng/mL vs 31.1 ng/mL; P = .001), together with an increased frequency of bone lesions (39% vs 8%; P = .002), organomegalies (39% vs 7%; P = .006), multilineage KIT mutation (100% vs 30%; P < .0001), and within multilineage cases, a greater number of cases with KIT-mutated myeloid and lymphoid cells (59% vs 22%; P = .03) (Table 2). Interestingly, HUMARA analysis showed a polyclonal X-chromosome inactivation pattern (XCIP) in purified BM MCs from 7 of 11 female patients who carried KIT D816V+ MSCs; by contrast, all 15 female patients analyzed who showed KIT D816V− MSCs had BM MCs with a clonal pattern of X-chromosome inactivation by HUMARA (Table 2).
Association between the presence of KIT D816V+ BM MSCs and patient outcome
Overall, 14 ISM cases showed disease progression: 8 ISM (57%) evolved to ASM, 4 to SSM (29%), and 2 to SM-AHNMD (14%) (an MPN and a B-cell non-Hodgkin lymphoma) (Table 3). Median time from disease onset to progression was 15 years. Except for a patient who progressed to ASM and displayed features associated with a moderate BM MC load (0.13% BM MCs and 52.6 ng/mL of serum baseline tryptase), all other patients displayed very high serum baseline tryptase levels (median, 275 ng/mL; range, 144-2036 ng/mL) and/or of BM MC percentages (median, 3.1%; range, 0.06%-18%) at progression. Of note, all ISM patients that progressed showed multilineage involvement of hematopoiesis by the KIT D816V mutation already at diagnosis.
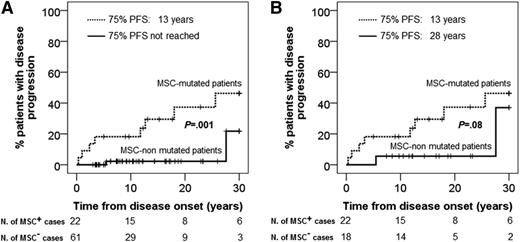
From those 14 ISM cases that showed progression, 11 belonged to the KIT D816V+ MSC group (11 of 22, 50%), whereas only 3 of 61 ISM cases with nonmutated MSCs showed disease progression (5%; P = .0005) (Table 2). Moreover, ISM patients with KIT D816V+ MSCs also showed a significantly (P < .001) shorter PFS vs patients who had nonmutated MSCs (75% PFS of 13 years vs not reached; P < .001) (Figure 1A) with significantly lower PFS rates at 10 years (82% ± 8% vs 98% ± 2%; P = .003), 20 years (63% ± 12% vs 98% ± 2%; P < .001), and 30 years (54% ± 13% vs 78% ± 18%; P = .001) (Table 4).
PFS of ISM patients classified according to the presence (dotted line) vs absence (full line) of the D816V KIT mutation in BM MSCs. (A) PFS from disease onset for all (n = 83) patients analyzed. (B) Analysis of PFS is restricted to patients (n = 40) with multilineage involvement of hematopoiesis by the KIT D816V mutation with (n = 22) or without (n = 18) D816V KIT-mutated MSCs.
PFS of ISM patients classified according to the presence (dotted line) vs absence (full line) of the D816V KIT mutation in BM MSCs. (A) PFS from disease onset for all (n = 83) patients analyzed. (B) Analysis of PFS is restricted to patients (n = 40) with multilineage involvement of hematopoiesis by the KIT D816V mutation with (n = 22) or without (n = 18) D816V KIT-mutated MSCs.
In order to determine whether the presence of KIT D816V+ MSCs would be a better predictor for disease progression than the occurrence of multilineage involvement of BM hematopoiesis by the KIT mutation, we further restricted the comparison between patients with (n = 22) vs without (n = 18) KIT D816V+ MSCs to multilineage cases (n = 40). Once again, ISM cases with KIT D816V+ MSCs more frequently had disease progression (50% vs 17%; P = .04) (Table 2). The adverse impact of having KIT D816V+ MSCs also translated into progressively shorter PFS rates (vs ISM cases having nonmutated MSCs) at 10 years (82% ± 8% vs 94% ± 5%), 20 years (63% ± 12% vs 94% ± 5%), and 30 years (54% ± 13% vs 63% ± 26%), although differences only reached marginal statistical significance at 20 years and 30 years (P ≤ .08) (Table 4). Despite the above differences in PFS rates, no statistically significant differences were found as regards overall survival between patients with vs without KIT D816V–mutated MSCs (overall survival rate at 10 years of 93.8% vs 100%, respectively). This is probably due to the fact that at the moment of closing this study, only 3 of 83 patients (3.6%) had died 7, 13, and 40 years after disease onset. Of note, 2 of 3 cases had multilineage KIT mutation with KIT-mutated MSCs; the other patient having nonmutated MSCs died of sepsis 40 years after ISM disease onset.
Discussion
Presence of the somatic KIT D816V mutation1,44 in BM MCs is a molecular hallmark of adult SM45 and a (minor) diagnostic criterion of the disease.22,35,36 Additionally, detection of the KIT D816V mutation is thoroughly applied to establish the clonal nature of the disease,3,22,46 and it has also been used as a molecular marker to track the clonal origin of different hematologic cell lineages within a patient (ie, through the definition of MC-restricted vs multilineage involvement of hematopoiesis) and to establish the clonal relationship between SM MCs and the AHNMD tumor cells in SM-AHNMD.12,13,15,16,23,47,48 Around half of all 83 ISM cases here analyzed showed multilineage involvement of BM cells by the KIT D816V mutation and half of these multilineage cases also had KIT-mutated MSCs. Most interestingly, the presence of mutated MSCs in the BM of ISM patients was associated with features of more advanced disease, a greater rate of disease progression, and a shorter PFS. Of note, the greater frequency of multilineage KIT mutation among our cases vs previously published series from our group was due to the need for enough purified BM MSCs for further molecular analyses, as discussed in “Methods.”
Interestingly, purified pathological KIT D816V+ BM MCs from a significant proportion of female ISM cases here analyzed showed a “polyclonal” XCIP, despite these cells most likely have a “clonally”-related origin. The cellular mosaicism resulting from the analysis of the XCIP in females has long been used as a marker to investigate clonal development and relationship among distinct cell compartments in different human hematopoietic disorders19,49,50 ; thus, a polyclonal XCIP in the absence of other genotypic markers (ie, KIT D816V) could be interpreted as an accumulation of otherwise reactive, nonclonal MCs. The apparent discrepancy observed between the genotypic clonality defined by the KIT D816V mutation and the polyclonal XCIP could be explained either by a hypothetical reactivation of the inactive X-chromosome51 at any stage after the occurrence of the mutation in a committed HPC, or by the emergence of the KIT mutation in an uncommitted precursor/stem cell at relatively early stages of development (ie, embryogenesis), prior to the inactivation of the X-chromosome during hematopoiesis. Because in human somatic cells XCIP appears to be rather stable,52 the second hypothesis seems most feasible. Thus, occurrence of the KIT D816V mutation during ontogeny would potentially target an early precursor/stem cell leading to multiple (instead of 1) involved HPCs; most likely, this would more frequently lead to multilineage involvement of hematopoiesis (and potentially also other tissues) by the KIT mutation. In line with this hypothesis, all ISM cases that displayed a polyclonal XCIP for their BM MCs also had multilineage involvement of hematopoiesis by the KIT mutation. These findings support previous observations in advanced SM about the origin of clonal MCs in a pluripotent HPCs with the ability to differentiate to other non-MC myeloid and even lymphoid lineages.12-16,45 Most interestingly, this polyclonal XCIP found across multilineage ISM cases was restricted to two-thirds of the ISM female patients carrying KIT-mutated MSCs, while absent among ISM female patients with wild-type KIT MSCs. MSCs are multipotent mesodermal progenitor cells present in the BM stroma which diverged from the hematopoietic lineages early during embryogenesis28 ; thereby, these results would support the notion that in these patients, the KIT mutation could have been acquired in a common pluripotent progenitor cell early during ontogeny, prior to differentiation into MSCs and HPCs. This hypothesis is also consistent with a report of 2 monozygotic twins with adult-onset ISM carrying the somatic KIT D816V mutation53 who both presented a mosaic pattern for the KIT mutation compatible with multilineage involvement of hematopoiesis. It is noteworthy that despite the (potentially) very early acquisition of this mutation during ontogeny, onset of typical clinical symptoms of mastocytosis was delayed to their middle adulthood, with 1 of the twins progressing from ISM to an SM-AHNMD 30 years after skin lesions had appeared53 ; this is in line with our observations and those of other groups.7,54
As a consequence of the occurrence of the KIT mutation in a common mesodermal ancestor of MSCs and HPCs, a mosaic of the mutated pluripotent precursor cell progeny would also carry the KIT genetic alteration, including both myeloid and lymphoid committed HPCs, as found in our cases. The more frequent (simultaneous) involvement of myeloid and lymphoid cells observed among ISM cases with mutated vs wild-type BM MSCs may be due to a greater number of involved HPCs and the more extended involvement of hematopoiesis in the former vs the latter group. In turn, the existence of a subset of MSC-mutated ISM patients showing only involvement of myeloid BM cells could be explained by preferential signaling of the mutated KIT in HPCs to the myeloid rather than the lymphoid lineages, the significantly different production and renewal rates of mature lymphocytes vs monocytes and neutrophils (longer vs shorter turnover times, respectively), or both.55-58 In addition, occurrence of a sporadic KIT D816V mutation that constitutively activates the stem cell factor/KIT signaling pathway could lend those CD117+ precursor cells with a proliferative and/or survival advantage. Thereby, the ability of a KIT D816V+ myeloid-committed HPC to colonize the patient’s BM will be faster than that of the mutated lymphoid-committed precursors, which would not have a proliferative advantage because of the activating KIT D816V mutation, due to loss of CD117 expression early during commitment to the lymphoid lineage.59 In turn, the presence of the KIT D816V mutation in BM MSCs should impair their function and potentially affect their role in supporting hematopoiesis and bone turnover. Even though we have not found any evidence of differences in bone lesions between patients with vs without mutated MSCs, purified CD105+/CD13high/CD45− BM MSCs from SM patients showed slower growth in vitro vs MSCs from healthy donors.39 These results are in line with recent observations by Nemeth et al who described the presence of abnormal MSCs with slow proliferation, signs of senescence, and impaired osteogenic function (vs normal MSC colonies) in SM patients.60 Of note, these authors did not find the KIT D816V mutation in cultured BM MSCs from any of the 5 SM patients they analyzed. The apparent discrepancy between these findings and our observations could be due to the relatively low number of patients analyzed or to a preferential growth of normal vs mutated MSCs after medium to long-term in vitro culture. In this regard, our results also showed that although the KIT D816V mutation was initially detected in cultured (purified) CD105+/CD13high/CD45− MSCs, it became negative in vitro after 3 culture passages, further supporting a survival/proliferative advantage in vitro for normal vs KIT-mutated MSCs from ISM patients.
Overall, one might expect that the greater the number of KIT-mutated HPCs, the greatest level of BM involvement by the KIT mutation, which would most likely contribute to pave the way for secondary (driver) genetic lesions and more frequent disease progression. In line with this, all cases having KIT-mutated MSCs also showed multilineage KIT mutation with a greater frequency of myeloid plus lymphoid (vs only myeloid) involvement of hematopoiesis. Moreover, such MSC-mutated ISM cases showed a greater frequency of progression to more advanced disease and shorter PFS rates, together with significantly greater levels of BM MC infiltration and serum baseline tryptase, and a greater frequency of organomegalies and bone lesions already at diagnosis. Of note, the rate of disease progression among multilineage cases was also significantly higher for patients with KIT-mutated vs nonmutated MSCs. Altogether, these results are consistent with recent observations in a mouse model with conditional expression of a constitutively active D814V-mutated KIT which showed a greater MC disease severity when the mutation was expressed in undifferentiated HPCs vs (only) more mature cells.61 In addition, our findings might also contribute to explain why ISM patients with MC-restricted KIT mutation in the BM have a normal life expectancy and very rarely progress to more aggressive disease.7 Of note, here, we reported a higher rate of progression of ISM patients than that observed in previous (large) series of ISM patients7,54,62 ; this is probably due to the preferential selection of cases with multilineage involvement of hematopoiesis by the KIT D816V mutation vs MC-restricted KIT D816V mutation because the latter cases typically showed very low rates of disease progression7 and/or a longer follow-up in this vs other previously reported series.
Overall, the above findings suggest that the clinical impact of constitutive activation of the stem cell factor/KIT signaling pathway critically depends on the stage of development at which the somatic KIT D816V mutation has been acquired, and the extent of involvement of the CD34+ HPC compartment (and therefore of the whole hematopoietic compartment). Thus, occurrence of the KIT mutation in an early progenitor cell during ontogeny will potentially lead to greater involvement of hematopoiesis, providing clonal cells an increased probability of acquiring secondary (driver) mutations/genetic alterations, particularly after long periods of time9 ; such genetic lesions might more frequently lead to progression and/or transformation of ISM to more severe disease and/or to the development of secondary myeloid (most frequently) and lymphoid neoplasias. Of note, acquisition and maintenance of these secondary mutations could be facilitated by the antiapoptotic and survival pathways which are differentially activated within the pathological MCs due to the KIT D816V mutation,25,27 in association with the more immature immunophenotype of BM MCs from patients carrying multilineage vs MC-restricted KIT D816V mutation.37 In line with this hypothesis, it has been recently shown in a murine model of SM, as well as among advanced SM patients,63 that coexistence of the KIT D816V mutation and loss of function of TET2 (or other mutations) in progenitor cells causes a more aggressive phenotype, typically mimicking advanced disease (ie, ASM).64
In summary, the results here presented demonstrate the occurrence of the KIT D816V mutation in BM MSCs from a substantial fraction of ISM patients that systematically showed multilineage involvement of hematopoiesis, in association with a greater risk for disease progression and shorter PFS. These findings suggest that among ISM cases, occurrence of the KIT D816V mutation in an earlier precursor cell is associated with a poorer outcome.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the Fondo de Investigaciones Sanitarias (grant PI11/02399, Fondo Europeo de Desarrollo Regional [FEDER]) and Red Temática de Investigación Cooperativa en Cancer (RTICC; grant RD12/0036/0048, FEDER) of the Instituto de Salud Carlos III (Ministry of Economy and Competitivity, Madrid, Spain); from Fundacion Ramon Areces (Madrid, Spain; grant CIVP16A1806); from Fundación Samuel Solorzano (Salamanca, Spain; grant FS/22-2014); and from Ayudas a Proyectos de Investigación en Salud de la Fundacion Mutua Madrileña 2014 and Asociacion Española de Enfermos de Mastocitosis (AEDM 2014). A. Mayado. was supported by RTICC. C.M. was supported by the Spanish Net on Aging and Frailty (RETICEF), Instituto de Salud Carlos III.
Authorship
Contribution: A.C.G.-M. designed the research, analyzed the data, interpreted results, made the figures, and wrote the paper; M.J.-A. performed experiments, analyzed the data, interpreted results, made the figures, and wrote the paper; I.A.-T. collected the samples, performed the clinical follow-up of the patients, and critically reviewed the paper; C.T., L.S.-M., J.I.M.-G., and A. Mayado performed experiments and critically reviewed the paper; C.M. performed MSC experiments and critically reviewed the paper; A. Matito performed the clinical follow-up of the patients and critically reviewed the paper; C.C. contributed with technical support and critically reviewed the paper; J.M.M. collected the samples and critically reviewed the paper; L.E. supervised the study, performed clinical follow-up of the patients, and critically reviewed the paper; and A.O. designed the research, supervised the study, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alberto Orfao, Centro de Investigación del Cáncer, Campus Miguel de Unamuno, 37007 Salamanca, Spain; e-mail: orfao@usal.es; and Andrés C. Garcia-Montero, Centro de Investigación del Cáncer, Campus Miguel de Unamuno, 37007 Salamanca, Spain; e-mail: angarmon@usal.es.
References
Author notes
A.C.G.-M. and M.J.-A. have contributed equally to this work and should both be considered as first author.