Key Points
AML-associated peripheral blood cytopenia is independent of bone marrow blast content, but strongly predicted by MPL expression on blast cells.
MPLhi blasts scavenge TPO from serum, causing insufficient cytokine levels.
Abstract
Although the molecular pathways that cause acute myeloid leukemia (AML) are increasingly well understood, the pathogenesis of peripheral blood cytopenia, a major cause of AML mortality, remains obscure. A prevailing assumption states that AML spatially displaces nonleukemic hematopoiesis from the bone marrow. However, examining an initial cohort of 223 AML patients, we found no correlation between bone marrow blast content and cytopenia, questioning the displacement theory. Measuring serum concentration of thrombopoietin (TPO), a key regulator of hematopoietic stem cells and megakaryocytes, revealed loss of physiologic negative correlation with platelet count in AML cases with blasts expressing MPL, the thrombopoietin (scavenging) receptor. Mechanistic studies demonstrated that MPLhi blasts could indeed clear TPO, likely therefore leading to insufficient cytokine levels for nonleukemic hematopoiesis. Microarray analysis in an independent multicenter study cohort of 437 AML cases validated MPL expression as a central predictor of thrombocytopenia and neutropenia in AML. Moreover, t(8;21) AML cases demonstrated the highest average MPL expression and lowest average platelet and absolute neutrophil counts among subgroups. Our work thus explains the pathophysiology of peripheral blood cytopenia in a relevant number of AML cases.
Introduction
Acute myeloid leukemia (AML) is a clonal proliferative disorder of hematopoietic stem and progenitor cells, causing death in the majority of patients within 5 years.1 AML induces profound impairment of nonleukemic hematopoiesis, leading to peripheral blood cytopenia, a major cause of AML-associated morbidity and mortality. Although the cytogenetic and molecular alterations of AML cells are being studied in detail,2 the mechanisms by which blasts interact with elements of normal hematopoiesis to cause cytopenia remain largely unexplored. It is clinically recognized that even with similar levels of bone marrow infiltration of hematopoietic malignancies, peripheral blood (PB) cytopenia varies widely. In chronic lymphocytic leukemia (CLL), normal peripheral blood cells are frequently not massively altered, although infiltration of CLL cells might exceed 50% of bone marrow (BM) cellularity. In contrast, PB cytopenia is more frequently observed in AML, even when blast percentages do not exceed 50% of BM cellularity. Nevertheless, the prevailing assumption is that PB cytopenia is a result of bone marrow nonleukemic hematopoiesis displacement.
Nonmalignant hematopoiesis is tightly regulated by cytokines.3 Among them, thrombopoietin (TPO) acts through its receptor MPL as the master regulator of megakaryopoiesis,4,5 but also exerts upstream effects on hematopoietic stem cells (HSCs), which express MPL.6-8 Systemic TPO levels are in part controlled by MPL receptor-mediated cytokine-scavenging, with platelets and megakaryocytes representing the major scavengers in a healthy organism,9,10 and in part by sensing of senescent platelets by the Ashwell-Morell receptor in the liver.11 Previous studies have demonstrated that, in a subset of cases, AML blasts can also express MPL12 and that circulating thrombopoietin may serve as an in vivo growth factor for blasts.13 We thus hypothesized that in a subset of AML cases, blasts might decrease TPO concentration by way of MPL mediated scavenging and therefore contribute to PB cytopenia development.
Study design
Patients
The initial single-center cohort comprised 223 patients with non-M3 AML. The validation multicenter cohort comprised 437 independent patients with non-M3 AML or refractory anemia with excess blasts (International Prognostic Scoring System score >1.5). Basic characteristics of the cohorts are described in supplemental Table 1, available on the Blood Web site.
Xenograft experiments
One million human BM blast cells from patients with newly diagnosed MPLhi or MPLlo AML were intrahepatically injected into sublethally irradiated human cytokine knock-in mice.14 Sixteen to 24 weeks after transplantation, AML engraftment was determined by flow cytometry, and human TPO (huTPO) serum concentration was measured using a bead-based multiplex assay.
Gene expression profiling analysis
Analysis of microarray expression data from dataset GSE1446815 was performed using the server-hosted version of Gene Pattern (http://genepattern.broadinstitute.org).
For further details, see supplemental Methods.
Results and discussion
Analysis of 223 patients with newly diagnosed AML at a single center revealed lack of relevant correlation between initial BM blast count and hemoglobin level, platelet count, and absolute neutrophil count, regardless of whether blast count was determined by aspirate cytology or biopsy histopathology (Figure 1A; supplemental Figure 1). These findings indicate that mechanisms different from displacement of normal hematopoiesis dictate the severity of cytopenia in AML patients. Of note, this is consistent with recent studies in the mouse showing that HSC differentiation, but not the total number of HSCs, is impaired in a murine xenograft model of AML16 and that HSC quiescence is increased in the MLL-AF9 leukemia model.17
AML blasts can scavenge TPO by virtue of their MPL expression, leading to inadequate cytokine levels. (A) Peripheral blood counts are shown as a function of bone marrow blast count, in patients with newly diagnosed AML at a single medical center (N = 223). Pearson product-moment correlation coefficient is stated. (B) Scatter plot depicting relationship between serum TPO concentration and platelet count (N = 52). Pearson product-moment correlation coefficient is stated. Selected potential scavenger AML cases (low platelets, low TPO; group A) are highlighted as gray squares and control AML cases (group B) as white squares. (C) Waterfall plot shows MPL mean normalized expression in individual patients identified by their unique ID below; box-and-whiskers plot shows comparison between groups A and B. (D) Histograms show MPL mean fluorescent intensity (MFI) on blasts (red line) compared with lymphocytes as internal control (blue line). MFI ratio blast-to-lymphocyte is stated. The 2 upper panels show potential scavenger cases; the 2 bottom panels show controls. Data are representative of N = 12. (E) Scatter plot depicting correlation between flow cytometry MFI and quantitative polymerase chain reaction mean normalized expression (N = 12). (F) TPO clearance after 2 hours of incubation of cells in medium supplemented with 100 pg/mL TPO (N = 4-6 per group). One-way ANOVA, P = .001. (G) Schematic representation of in vivo xenotransplantation experiments. (H) Scatter plot depicts huTPO serum concentration in the xenotransplantation setting by donor-engraftment status. Average engraftment level in the respective groups is stated. N = 3 per group MPLhi and group MPLlo for primary AML samples/patients. N = 3-4 per group for murine recipients. One-way ANOVA, P < .0001. ANC, absolute neutrophil count.
AML blasts can scavenge TPO by virtue of their MPL expression, leading to inadequate cytokine levels. (A) Peripheral blood counts are shown as a function of bone marrow blast count, in patients with newly diagnosed AML at a single medical center (N = 223). Pearson product-moment correlation coefficient is stated. (B) Scatter plot depicting relationship between serum TPO concentration and platelet count (N = 52). Pearson product-moment correlation coefficient is stated. Selected potential scavenger AML cases (low platelets, low TPO; group A) are highlighted as gray squares and control AML cases (group B) as white squares. (C) Waterfall plot shows MPL mean normalized expression in individual patients identified by their unique ID below; box-and-whiskers plot shows comparison between groups A and B. (D) Histograms show MPL mean fluorescent intensity (MFI) on blasts (red line) compared with lymphocytes as internal control (blue line). MFI ratio blast-to-lymphocyte is stated. The 2 upper panels show potential scavenger cases; the 2 bottom panels show controls. Data are representative of N = 12. (E) Scatter plot depicting correlation between flow cytometry MFI and quantitative polymerase chain reaction mean normalized expression (N = 12). (F) TPO clearance after 2 hours of incubation of cells in medium supplemented with 100 pg/mL TPO (N = 4-6 per group). One-way ANOVA, P = .001. (G) Schematic representation of in vivo xenotransplantation experiments. (H) Scatter plot depicts huTPO serum concentration in the xenotransplantation setting by donor-engraftment status. Average engraftment level in the respective groups is stated. N = 3 per group MPLhi and group MPLlo for primary AML samples/patients. N = 3-4 per group for murine recipients. One-way ANOVA, P < .0001. ANC, absolute neutrophil count.
We thus hypothesized that an altered cytokine milieu may contribute to cytopenia. We chose to study TPO, as this cytokine is known to influence both megakaryopoiesis and upstream hematopoietic stem and progenitor cells.4,7,18 In the steady state, receptor-mediated scavenging results in a negative correlation between serum TPO concentration and platelet count.9,10 When we examined this relationship in AML, TPO levels did not follow the expected negative correlation with platelet counts (Figure 1B). Comparison with historic controls with nonhematopoietic malignancy and chemotherapy-induced thrombocytopenia19 revealed that the lack of correlation was driven by AML cases with severe thrombocytopenia that had lower-than-expected TPO serum concentration.
We next contrasted MPL expression on blasts in AML cases with severe thrombocytopenia and low TPO concentration (potential scavenger cases, group A; Figure 1B) to cases with TPO levels adequate for the degree of cytopenia (group B; Figure 1B). Both surface flow cytometry and quantitative polymerase chain reaction demonstrated higher MPL expression in potential scavenger cases (Figure 1C-D; supplemental Figure 2), with both measurements showing strong concordance (Figure 1E).
To mechanistically determine whether this difference in expression translates into increased serum TPO clearance, we first incubated AML blasts with high (MPLhi) or low (MPLlo) receptor expression in serum containing recombinant huTPO at a high physiologic concentration. After 2 hours, TPO clearance reached 45 pg/106 cells in wells containing MPLhi blasts compared with only 4 pg/106 cells in wells with MPLlo blasts and no TPO clearance when control lymphocytes were used (Figure 1F). To test whether this holds true in vivo, we transplanted humanized mice expressing huTPO under the endogenous murine promoter14 with MPLhi or MPLlo AML blasts (Figure 1G). Although AML engraftment levels in the BM were similar at 16 to 24 weeks after transplantation, mice that had engrafted with MPLhi AML showed significantly lower huTPO concentrations in the serum compared with their littermates that were engrafted with MPLlo AML (Figure 1H). Together, this confirms that AML blasts can lower TPO levels by virtue of their MPL expression.
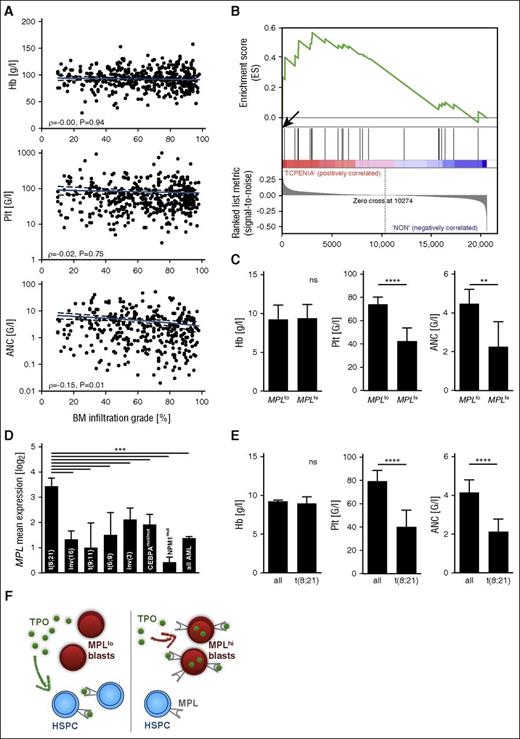
We subsequently tested our findings in an independent patient population. We confirmed lack of correlation between initial bone marrow blast count and cytopenia in 437 AML cases of a multicenter Dutch-Belgian-Swiss study cohort (Figure 2A). Unbiased ranked gene list correlation analysis of whole genome microarray data in this cohort proved highly significant enrichment of the MPL transcript in patients with severe thrombocytopenia compared with patients with average platelet counts (rank 27 of 20 606 genes, P < .002; supplemental Table 2). Gene set enrichment analysis20 showed concomitant enrichment of TPO pathway genes (Figure 2B; supplemental Table 3; supplemental Figure 3) and MPL transcriptional targets (supplemental Table 4; supplemental Figure 3), indicating increased signaling activity in the pathway. Of note, no other cytokine receptors were represented among the 100 most highly overexpressed genes (supplemental Table 2). Accordingly, MPLhi status was highly predictive of more severe thrombocytopenia and neutropenia at time of AML diagnosis; however, there was no correlation between MPL transcript level and the degree of anemia (Figure 2C).
MPL expression is predictive of cytopenia in a multicenter AML cohort. (A) Peripheral blood counts are shown as a function of bone marrow blast count in patients with newly diagnosed AML/refractory anemia with excess blasts from the Dutch-Swiss-Belgian HOVON study group (validation cohort) (N = 437). Pearson product-moment correlation coefficient is stated. (B) Gene set enrichment analysis of the BIOCARTA_TPO_PATHWAY gene set (supplemental Table 3) in AML patients with severe thrombocytopenia (0-20 G/L) at diagnosis compared with patients with platelet counts between 50 and 100 G/L (average in AML, 79 G/L) (N = 100). Enrichment score (ES), 0.56; significant at P < 5%. Arrow marks MPL at position 27 in ranked gene list. (C) Peripheral blood counts at diagnosis are shown as a function of MPL status by microarray. P < .0001 (platelet count), P = .0077 (ANC). (D) MPL mean expression as a function of AML (cytogenetic or molecular) subgroup according to the WHO 2008 classification. One-way ANOVA, P < .0001. (E) Initial peripheral blood counts in patients with AML with t(8;21) from both cohorts (N = 32) compared with all other AML patients. P < .0001 (platelet count, ANC). (F) Cartoon depicting the proposed mechanism leading to cytopenia in MPLhi AML (right), contrasted with MPLlo AML (left).
MPL expression is predictive of cytopenia in a multicenter AML cohort. (A) Peripheral blood counts are shown as a function of bone marrow blast count in patients with newly diagnosed AML/refractory anemia with excess blasts from the Dutch-Swiss-Belgian HOVON study group (validation cohort) (N = 437). Pearson product-moment correlation coefficient is stated. (B) Gene set enrichment analysis of the BIOCARTA_TPO_PATHWAY gene set (supplemental Table 3) in AML patients with severe thrombocytopenia (0-20 G/L) at diagnosis compared with patients with platelet counts between 50 and 100 G/L (average in AML, 79 G/L) (N = 100). Enrichment score (ES), 0.56; significant at P < 5%. Arrow marks MPL at position 27 in ranked gene list. (C) Peripheral blood counts at diagnosis are shown as a function of MPL status by microarray. P < .0001 (platelet count), P = .0077 (ANC). (D) MPL mean expression as a function of AML (cytogenetic or molecular) subgroup according to the WHO 2008 classification. One-way ANOVA, P < .0001. (E) Initial peripheral blood counts in patients with AML with t(8;21) from both cohorts (N = 32) compared with all other AML patients. P < .0001 (platelet count, ANC). (F) Cartoon depicting the proposed mechanism leading to cytopenia in MPLhi AML (right), contrasted with MPLlo AML (left).
Last, we tested if MPL expression was related to a cytogenetic or molecular AML subtype. Indeed, microarray analysis showed significantly higher MPL expression in cases of AML with t(8;21) than in any other subtype (Figure 2D), consistent with previous studies, which demonstrated functional relevance of TPO/MPL signaling to initiation and maintenance of this exact subtype of AML.21,22 Concurrently, these patients displayed significantly lower platelet counts (40 vs 83 G/L) and lower absolute neutrophil counts (2.3 vs 4.3 G/L) at diagnosis compared with all other AML cases (Figure 2E).
In summary, our study demonstrates that thrombocytopenia and neutropenia in AML are independent of bone marrow blast count but predicted by MPL expression on blasts. We show that MPLhi blasts can clear TPO in vitro and decrease its serum concentration in vivo, providing a molecular basis for severe thrombocytopenia and neutropenia observed in patients with MPLhi AML (Figure 2F). Why erythropoiesis is not affected in the same way warrants further discussion: although TPO is known to exert effects on the HSC level,6-8 data from clinical trials and in vitro studies involving MPL agonists support a model where there can be differential lineage effects favoring megakaryopoiesis and myelopoiesis.18,23 Of note, it is also very likely that additional factors, potentially acting in synergy with MPL, contribute to development of cytopenia, and especially anemia, in AML, which should be addressed in future studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to all patients and their physicians who participated in the study. The authors thank all members of the Manz group for helpful discussion and the Clinical Hematology laboratory at University Hospital Zurich for expert technical assistance.
This study was supported by the Swiss National Science Foundation (310030_146528/1) and the Clinical Research Priority Program “Human Hemato-Lymphatic Diseases” of the University of Zurich (M.G.M.) and a Hanne-Liebermann fellowship (P.J.R.).
Authorship
Contribution: P.J.R., H.T., and M.G.M. designed the study; P.J.R., C.C.W., J.M.E., J.S.G., P.J.M.V., and B.L. collected and analyzed patient data; P.J.R., J.M.E., and K.F. performed experiments; P.J.R., J.M.E., H.T., and M.G.M. interpreted data; P.J.R. wrote the manuscript; M.G.M. supervised the study and revised the manuscript; and all authors have seen and approved the final version of the manuscript before submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus G. Manz, Hematology, University Hospital and University of Zurich, Raemistr 100, CH-8091 Zurich, Switzerland; e-mail: markus.manz@usz.ch.