Abstract
Background
Targeting new antigens in chronic lymphocytic leukemia (CLL) and lymphoma may increase flexibility in the clinic and help circumvent resistance. The tetraspanin CD37 domain mediates transduction of survival and apoptotic signals (Lapalombella et al.,Cancer Cell, 2014), and has been clinically validated by recent trials of otlertuzumab (TRU-016) in CLL and Non-Hodgkin Lymphoma . Ligation of CD37 by this reagent simultaneously induced pro-apoptotic signaling and inhibited pro-survival signaling of phosphoinositide 3-kinase δ (PI3Kδ), which introduces a unique opportunity to use combination strategies employing activation of CD37 and inhibition of PI3Kδ. A new agent BI 836826 is an Fc-engineered anti-CD37 IgG1 that displays improved effector activities as well as crosslinker-independent direct cytotoxicity. We have evaluated the efficacy of BI 836826 combined with the PI3Kδ-selective inhibitor idelalisib in diffuse large B-cell lymphoma (DLBCL) cell lines and primary human CLL B-cells in the University and then by industry to validate the synergistic finding initially reported.
Methods Cell viability assays usedCellTiterGlo to measure inhibition of antibody, isotype control, idelalisib or a combination of antibody and compound over 72h in culture. The cell viability of vehicle is measured at the time of dosing (T0) and after seventy-two hours (T72). A GI reading of 0% represents no growth inhibition, GI 100% represents complete growth inhibition, and a GI 200% represents complete death of all cells in the culture well. Annexin V-FITC and propidium iodide measure by flow cytometry was used to assess enhanced killing of primary CLL cells, with incubation of BI 836826 (0.1 µg/mL) and/or idelalisib (1 µM) at 37°C for 24 hours. Trastuzumab included as a non-specific IgG1 control. Data was reported as percentage of viable cells (Annexin V negative, PI negative) normalized to untreated control.
Results
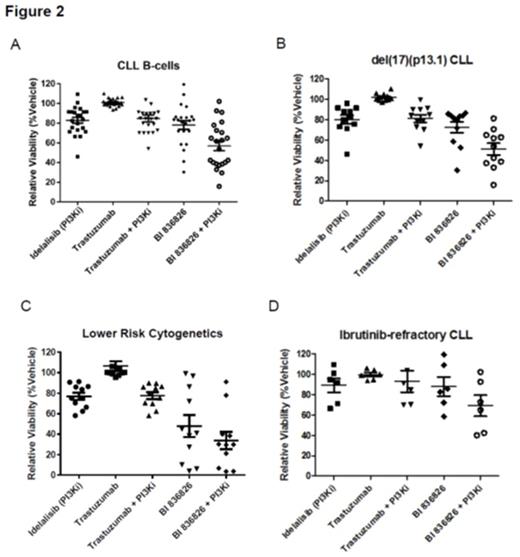
DLBCL cell lines were variably sensitive to single agent BI 836826. In most of the cell lines tested, the cell viability was inhibited by 40%-50% with BI 836826 in the concentration range of 1-1000 ng/mL (Figure 1A). A synergistic effect was noted in several DLBCL cell lines when BI 836826 was combined with idelalisib. When the maximal effect of BI 836826 was greater than isotype control (GI% > 12, dotted line) and the effect of idelalisib showed a GI50 < 1uM, 3/5 cell lines showed synergy in combination (red dot, Figure 1B). A shift in the EC50of idelalisib can be seen with the addition of increasing amounts of BI 836826 (Figure 1C). In primary CLL B-cell cultures, 1 µM idelalisib displayed weak single agent activity following 24-hour incubation. The cytotoxicity of BI 836826 at 0.1 µg/mL was more variable, although treatment of samples from most CLL patients resulted in 20-50% B-cell death. The combination of these 2 agents resulted in enhanced cytotoxic activity (Figure 2A), and this effect was not attenuated by the presence of del(17)(p13.1), as there was no significant difference in cytotoxicity against these cells compared to those with lower risk cytogenetics (Figure 2B,C). Additionally, the combination was beneficial in CLL B-cells isolated from patients who were refractory to ibrutinib (Figure 2D).
Conclusions
This collaborative industry and academic endeavor with cross validation of initial mechanistic studies of synergy between CD37 and idelalisib demonstrates that addition of idelalisib to BI 836826 augments cytotoxicity against DLBCL cell lines and primary human CLL B-cells in an additive-to-synergistic manner. In addition, it maintains efficacy against CLL B-cells with del(17)(p13.1) and those from ibrutinib-refractory patients. Further exploration of this therapeutic strategy in clinical trials is strongly warranted.
Jones:AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, LLC, an AbbVie Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Awan:Innate Pharma: Research Funding; Pharmacyclics: Consultancy; Novartis Oncology: Consultancy. Grosmaire:Gilead: Employment. Jones:Gilead: Employment. DiPaolo:Gilead: Employment. Tannheimer:Gilead Sciences: Employment. Heider:4Boehringer Ingelheim RCV: Employment.
Author notes
Asterisk with author names denotes non-ASH members.