Abstract
Introduction: BK virus (BKV) seropositivity is highly prevalent (80-90%) in healthy adults, but BKV rarely causes significant clinical disease. However, immunologic control of BKV is often compromised after allogeneic hematopoietic stem cell transplantation (HSCT) and BKV reactivation can cause clinical disease, including hemorrhagic cystitis, in 5-68% of patients in the first year after HSCT. Immune reconstitution of BKV-specific T cells after HSCT has not been well defined, and the role of BKV-specific T cells in response to clinical infection has not been studied.
Methods: From the DFCI 2010-2011 HSCT cohort (Rorije et al, BBMT 2014), 33 adult patients were selected who had urinary symptoms, were tested for urine BKV by PCR, and were diagnosed with BKV disease (cases; n=16) or did not have BKV reactivation (controls; n=17). Cases and controls were matched for cyclophosphamide use, acute GvHD and stem cell source. BKV-specific T cells were analyzed in 180 prospectively cryopreserved peripheral blood mononuclear cells (PBMC) samples (average 5.5/patient) across several timepoints (pre-HSCT and 1, 3, 6, 9, 12, 18 and 24 months post-HSCT) using cytokine flow cytometry (CFC). PBMC were stimulated for 6 hours with overlapping 15-mer peptides derived from BKV LT and VP1 proteins in the presence of brefeldin A, monensin and anti-CD28/49d. Results were compared with no-pepmix negative controls and Staphylococcal enterotoxin B positive controls. After stimulation, cells were stained with fluorochrome-conjugated antibodies specific for CD3, CD4, CD8, CD45RA, CCR7, CD57, CD107a, IFNγ, TNFα, IL-2, perforin (PERF) and granzyme B (GZMB). Laboratory assessments were blinded to clinical disease status.
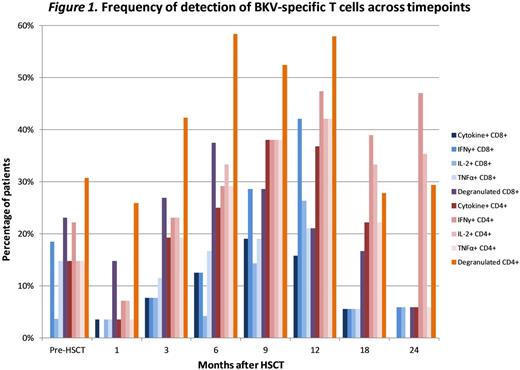
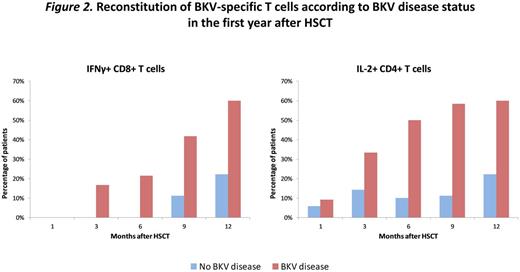
Results: Median age of the cohort was 51 years (IQR; 39-61); 79% were male and acute GvHD occurred in 64%. Conditioning included cyclophosphamide in 46% and ATG in 24%; cell source was peripheral blood stem cells in 81%, cord blood in 15% and bone marrow in 3%. Median time from HSCT to BKV testing was 60 days (23-181); median time to first positive test was 79 days (26-196) in BKV+ cases. Median duration of symptoms was 53 days (11-92) in cases, compared to 5 days in controls. The percentage of patients with BKV-specific T cells across timepoints is summarized in Figure 1. The frequency of BKV-specific degranulating (CD107a) T cells peaked at 6mo, where they could be detected in 37.5% and 58.3% of patients by reactivity of CD8+ and CD4+ T cells, respectively. Detection of BKV-specific T cells producing any cytokines (cytokine+) peaked at 9 mo (CD8+ in 19% and CD4+ in 38.1% of patients). BKV-specific IFNγ+, IL-2+, and TNFα+ T cells all peaked at 12mo after HSCT. Subsequently, detection of BKV-specific CD8+ T cells declined sharply while BKV-specific CD4+ T cells were maintained at higher levels 24 months after HSCT. Comparing BKV-specific immune responses in cases and controls, IFNγ+ CD8+ cells reconstituted faster in patients with clinical disease (first detected at 3 months vs. 9 mo), and were present in more patients at 9 months: 41.7% vs. 11.1%. A similar trend was noted for IL-2+ CD8+, IFNγ+ CD4+, and IL-2+ CD4+ T cells (Figure 2). BKV-specific CD8+ T cells were predominately central memory (CM) and terminally differentiated effector memory and their cytokine profile increased gradually after transplant; 19% produced at least two cytokines at 1 mo, compared to 71% at 12 mo. BKV-specific CD4+ T cells were predominately CM and effector memory and their cytokine profile also increased after transplant; 3.5% produced at least two cytokines at 1 mo, compared to 35% at 12 mo and 69.5% at 24 mo. PERF and/or GZMB were detected in 45-74% of BKV-specific CD107a CD8+ cells. CD107a CD8+ cells also produced IFNγ.
Conclusion: BKV-specific T cells gradually reconstitute in the first 9 months after allogeneic HSCT. 30-40% of patients with clinical symptoms and 50-60% of patients with confirmed BKV disease had detectable BKV-specific T cells but BKV-specific immunity recovers more rapidly in patients with BKV disease. As CD8 and CD4 BKV-specific immunity recovers, T cells gradually become more functional with a memory phenotype. Our cohort is currently being expanded to assess the correlation between the development and expansion of BKV-specific T cells and clinical outcomes.
Koreth:kadmon corp: Membership on an entity's Board of Directors or advisory committees; takeda pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; amgen inc: Consultancy; LLS: Research Funding; prometheus labs inc: Research Funding; millennium pharmaceuticals: Research Funding. Armand:Otsuka: Research Funding; BMS: Consultancy, Research Funding; Infinity: Consultancy; Sequenta: Research Funding; Roche: Research Funding; Merck & Co., Inc.: Consultancy, Research Funding; Sigma Tau: Research Funding; Tensha: Research Funding. Soiffer:GentiumSpA/Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Ritz:Kiadis: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.