Abstract
Introduction: Children with Down syndrome (DS) have a 10-20 fold increased risk of acute lymphoblastic leukemia (ALL) and suffer significantly poorer outcomes due to increased relapse and treatment-related mortality. DS-ALL exhibits a distinct spectrum of cytogenetic alterations, with a lower incidence of common recurrent ALL alterations, a higher incidence of alterations involving CRLF2 and JAK2, and a higher proportion of cases lacking a known oncogenic driver compared to non-DS ALL. We hypothesized that identifying genetic drivers in DS-ALL will facilitate improved risk-based treatment stratification and lead to novel therapeutic targets that may be harnessed to improve outcomes in this vulnerable population.
Methods: We interrogated diagnostic tumor (bone marrow or peripheral blood) and matched remission ("normal") blood samples from a cohort of 63 DS-ALL cases (65% male; median age 5 years, range 1-21 years) obtained from Texas Children's Cancer Center and the Children's Oncology Group. Samples were selected to include ~1/3 CRLF2-rearranged (CRLF2-R) and ~2/3 CRLF2-wild-type (CRLF2-WT) cases. Whole exome sequencing (WES) and copy number analysis using the OmniExpress array were performed on both tumor and normal DNA and transcriptome sequencing (RNAseq) was conducted on tumor RNA, all using the Illumina platform and existing informatic pipelines at the Baylor College of Medicine Human Genome Sequencing Center. A median of >97% of targeted bases with at least 20x coverage (range 93.5-98.1%) was observed for WES, and an average of 55.4 million paired reads were generated per RNAseq.
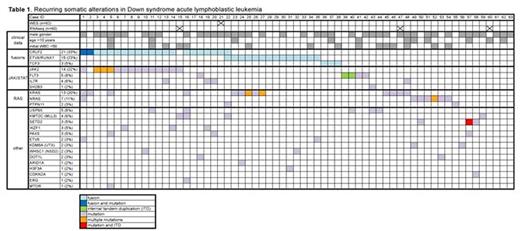
Results: Analysis of RNAseq data (n=60) using deFuse and SOAPfuse algorithms revealed known ALL-associated gene rearrangements in 37/60 (62%) of cases, including CRLF2-R (21/60, 35%) involving P2RY8 (n=17) or IGH (n=4); ETV6-RUNX1 fusions (14/60, 23%) and TCF3 fusions (PBX1 x 2 and a novel in-frame fusion with FLI1 x 1). WES (n=62) identified a median of 19 nonsilent somatic mutations per case (range 6-228). We did not find evidence of somatic or germline mismatch repair gene mutations in the 3 cases with >50 somatic mutations. WES findings included recurrent activating hotspot mutations in JAK2 (predominantly p.R683G/S) in 14 cases (23%) and CRLF2 (p.F232C) in 2 cases, all of which also had CRLF2-R. Somatic RAS pathway mutations (13 KRAS, 7 NRAS, and 2 PTPN11) were found in 20/62 cases (32%), 4 CRLF2-R and 16 CRLF2-WT, including 2 cases also harboring JAK2 activating mutations. Recurrent mutations in epigenetic or chromatin remodeling genes (e.g. KMT2C, SETD2, KDM6A, WHSC1, DOT1L) were observed in ~25% of patients, as were mutations in other known ALL genes (e.g. FLT3, IL7R, IKZF1, PAX5). Interestingly, inactivating mutations in the deubiquitinase USP9X, also recently reported in acute promyelocytic leukemia, were identified in 5/62 (8%) cases. Evaluation for internal tandem duplications (ITDs) demonstrated typical in-frame events in FLT3 (n=2) and an out-of-frame ITD in SETD2. Copy number analysis revealed frequent losses in cell cycle genes (CDKN2A/B, BTG1, RB1) and other known B-cell development genes (IKZF1, PAX5). Other putative candidate alterations detected by RNAseq, WES, and array are currently being validated by orthogonal platforms. We observed multiple subclonal driver events in >10% of patients, including one case with CRLF2-R and mutations in CRLF2, JAK2, KRAS, and NRAS; and a second case with CRLF2 and ETV6-RUNX1 rearrangements and a PTPN11 hotspot mutation. Six cases exhibited 2 different JAK2, KRAS, or NRAS mutations: in all four cases where evaluation was feasible, these mutations were present on different sequencing reads, suggesting the existence of distinct subclones. Clinical features (gender, age, initial white blood count) did not appear to co-cluster with any of the recurrent alterations.
Conclusions: These findings detail the landscape of genomic and transcriptomic alterations in the largest cohort of DS-ALL comprehensively characterized to date. Our data confirm the frequency and occasional polyclonal nature of JAK2 and RAS pathway mutations, and demonstrate that mutations in these pathways are not mutually exclusive. We also identified several novel, recurrent mutations and fusions. Future analyses of additional cohorts will be needed to assess recurrence frequency and prognostic impact of these alterations.
Schore:Baxalta: Honoraria; Millennium Pharmaceuticals, Inc: Research Funding; Onyx/Amgen: Research Funding; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.