Abstract
Background: Cellular senescence has been recognized as a failsafe mechanism against hyperproliferation and might thus be induced by DNA replicative stress and oncogenic signaling, commonly termed oncogene-induced senescence (OIS). OIS has been described in several premalignant conditions such as colon adenomas and melanocytic nevi, with impaired OIS capabilities found in their malignant counterparts. Here, we analyze the possible impact of cellular senescence on malignant transformation in plasma cell disorders.
Methods: Bone marrow and soft tissue biopsies from 125 patients with different stages of plasma cell disorders (16 monoclonal gammopathy of undetermined significance (MGUS), 32 smoldering multiple myeloma (SMM), 56 symptomatic multiple myeloma (MM), 21 extramedullary MM) as well as from 10 healthy donors were analyzed. Expression of OIS associated proteins p16INK4A, p21Cip1/Waf1, p27Kip1, phospho-Chk2, the DNA double-strand break marker γH2AX, as well as the proliferation marker Ki67 were assessed on plasma cells by immunohistochemistry. Additionally, double staining experiments for p21 and Ki67 were performed applying immunofluorescence confocal microscopy. Levels of protein expression were compared between different disease stages using the Kruskal-Wallis test.
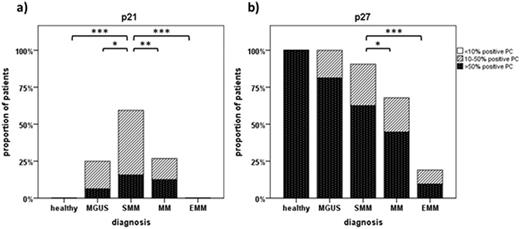
Results: A differential expression pattern was found for p21 in various stages of plasma cell disorders with peak expression of p21 in SMM compared to both healthy controls (p<0.001) and MGUS (p=0.02), as well as compared to symptomatic multiple myeloma (MM) (p=0.007) (see Figure 1a). Median p21 expression was 0.63% of plasma cells from healthy subjects, 6.67% in MGUS, 13.81% in SMM, 2.37% in MM, and 0% in EMM. Plasma cells of SMM patients expressing p21 were negative for Ki67 consistent with a potentially senescent phenotype. In contrast, p27 was highly expressed in healthy controls, MGUS and SMM but decreased significantly in MM patients (p=0.02) (see Figure 1b). p16 showed no nuclear expression in healthy controls, MGUS or SMM and was expressed only in few patients with MM. In addition, we found low expression of p21, p27 and phospho-Chk2 in extramedullary MM compared to medullary MM samples, accompanied by increased expression of γH2AX and high levels of proliferation (Ki67 58%).
Conclusions: We found indication of induction of OIS in SMM compared to symptomatic MM, mainly mediated by increased expression of p21. Further disease progression to extramedullary MM was characterized by almost complete absence of OIS markers and increased signs of DNA damage and proliferation. These observations are consistent with the hypothesis of OIS as a breakpoint mechanism against malignant transformation in plasma cell disorders and should be further explored mechanistically and as a possible therapeutic target.
Expression levels of p21 and p27in different stages of plasma cell disorders. Semiquantitative assessment of plasma cells positive for p21 (a) and p27 (b) is shown in healthy controls, MGUS, SMM, MM, and EMM patients. Significant differences in expression levels between cohorts are indicated by their respective p-values with * p-value < 0.05, ** < 0.01, *** < 0.001.
Expression levels of p21 and p27in different stages of plasma cell disorders. Semiquantitative assessment of plasma cells positive for p21 (a) and p27 (b) is shown in healthy controls, MGUS, SMM, MM, and EMM patients. Significant differences in expression levels between cohorts are indicated by their respective p-values with * p-value < 0.05, ** < 0.01, *** < 0.001.
Goldschmidt:Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Chugai: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium: Membership on an entity's Board of Directors or advisory committees, Research Funding; Onyx: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Raab:Novartis: Consultancy, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.