Abstract
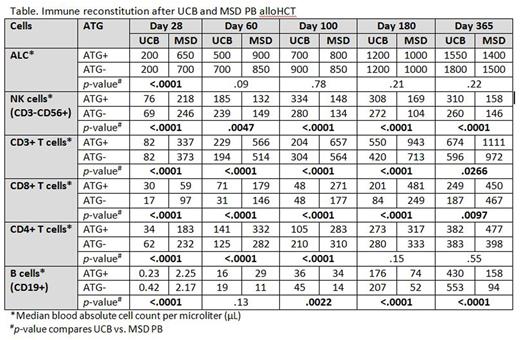
IR is a major obstacle for the successful use of alloHCT regardless of stem cell source. As alloHCT using a MSD is generally regarded as the gold standard, we evaluated the pace of IR in adult (age ≥18 years) recipients of HLA 0-2/6 mismatched UCB as compared to those transplanted with a MSD PB for hematological malignancy. In this prospective analysis we evaluated IR at days 28, 60, 100, 180 and 365 in 241 recipients of UCB (162 without ATG and 79 with ATG) and 151 recipients of MSD PB (108 without ATG and 43 with ATG) between 2004 and 2014. All patients received a non-myeloablative conditioning (NMAC) regimen consisting of fludarabine 200 mg/m2, cyclophosphamide 50 mg/kg and TBI200 cGy with equine ATG (90 mg/kg) if the patient had not had immunosuppressive chemotherapy in the prior 3 months or had a prior autologous transplant. Median age at transplant was 57.5 years (range, 18.8-73.3); 187 patients (47.7%) were alive at last follow up. Patient demographics, including disease risk index, were similar between the two stem cell sources except for a greater proportion of patients with leukemia in recipients of UCB (62.2% vs 52.3% in recipients of MSD PB). On day 28, the absolute lymphocyte count (ALC) was significantly lower after UCB allograft (p<0.01); however there were no differences in ALC thereafter (Table). Compared to MSD PB, UCB recipients had lower absolute numbers of NK-cells (CD3-CD56+) at day 28, but thereafter were significantly higher at all time points (p<0.01). Examining NK-cell subsets, including the CD56bright, CD56dim, KIR expressing NK-cells and adaptive NK (NKG2C+CD57+), UCB recipients recovered these subsets more rapidly after day 28 (all p<0.01). Conversely, UCB recipients had delayed T-cell recovery independent of ATG use, with lower numbers of T-cells (CD3+) and NK T-cells (CD3+CD56+) at all time points within 1-year of transplant compared to MSD PB recipients (p<0.03 at all times). These differences were mainly accounted for by the CD8+ T-cell subset that remained significantly lower in UCB recipients at all time points (p<0.01). CD4+ T-cells, however, were significantly lower than in recipients of MSD PB only for the first 100 days (p<0.01). Considering that UCB is composed of almost entirely naïve T-cells, we evaluated T-cell subsets. Overall, differences between UCB and MSD PB decreased over time after transplantation. The absolute numbers of both CD4+ and CD8+ naïve (CD45RA+ CD27+) T-cells were lower in recipients of UCB compared to MSD PB (p<0.001 at all times during the first year). CD4+ and CD8+ central memory (CD45RA-CD27+) and effector memory (CD45RA-CD27-) T-cells were also significantly lower in UCB recipients for the first 100 days (p<0.001). Notably, B-cells (CD19+) were higher in recipients of UCB after day 60 at all times (all p<0.01). ATG had little effect in recipients regardless of stem cell source after NMAC. Infection density analysis showed higher risk of bacterial (3.19 vs. 2.38; p=0.01) and viral (3.91 vs. 2.03; p<0.01) infections (per 1000 patient-days) in UCB vs. MSD PB recipients between days 28 and 365 after alloHCT, whereas the risk of fungal infections (0.19 vs. 0.40; p=0.04) was higher in MSD PB recipients. In multiple regression analysis, UCB was associated with significantly lower risk of chronic GVHD. Notably, incidence of transplant-related mortality, relapse and grade II-IV acute GVHD were similar with comparable disease-free and overall survival after 0-2 mismatched UCB and MSD PB alloHCT. In summary, UCBT recipients have significantly more rapid and robust NK-cell and B-cell recovery, but slower T-cell reconstitution, particularly CD8+ subsets. Effect of ATG was minimal. While slower T-cell reconstitution may predispose UCB recipients to certain infections, it has not hampered its graft-vs-malignancy effect, perhaps related to the robust NK-cell recovery. How IR after UCB and MSD PB compares with haploidentical and HLA-matched unrelated donors needs to be further explored.
Miller:Oxis Biotech: Consultancy, Other: SAB; Fate Therapeutics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.