Abstract
Introduction
Comorbidity prior to HCT predisposes to non-relapse mortality (NRM) and has been well described using the hematopoietic cell transplantation-comorbidity index (HCT-CI). Prior solid tumor automatically categorizes patients as high comorbidity whereas hematologic cancer different from the primary transplant indication is not scored (e.g., prior lymphoma in a patient with AML). No studies have explicitly characterized the risk from cancer comorbidity alone prior to HCT.
Methods
We retrospectively reviewed records of HCT patients 18 years and older who underwent first allogeneic HCT from 2009-2014. Solid tumors were defined according to the HCT-CI as any previous solid tumor malignancy excluding non-melanoma skin cancers. Hematologic (heme) malignancies were defined as any prior hematologic malignancy excluding the primary indication for HCT and excluding disease evolution. Cancer severity was also graded according to the Cumulative Illness Rating Scale-Geriatric (CIRS-G) as follows: 0: no malignancy, 1: cancer >10 years prior without sequelae, 2: cancer <5 years prior, 3: cancer requiring chemotherapy, radiation, hormonal therapy or surgery in the past 5 years, 4: recurrent malignancy of life-threatening potential.
Results
Among 356 first transplants with a median age of 53 years (range, 19-75), we found 59 total comorbid cancers among 54 patients (15% of total), with 26 hematologic and 33 solid cancers. Five patients had 2 malignancies (4 patients had both a solid and hematologic comorbid cancer). Breast (n=12/59; 20%) and prostate cancers (n=8/59; 14%) were the most common solid tumors; lymphoma was the most frequent comorbid hematologic neoplasm (n=14/59; 24%). More patients in the cancer comorbidity cohort were aged 50+ compared to the cohort without cancer comorbidity (83% vs 57%, P=<0.01). The majority underwent allografting for therapy-related myeloid neoplasm (n=35, 65%). Initial cancer staging could only be determined in 47% of patients. In the solid tumor cohort, 29 patients (89%) had their comorbid cancer in remission prior to HCT compared to 17 (65%) in the heme malignancy cohort. Prior treatments for cancer were as follows: 57% received surgery, 65% received chemotherapy, 39% received radiation, and 11% received hormonal therapy. Only 12 (22%) patients received non-chemotherapy regimens alone (i.e. surgery and/or hormonal therapy). There were no relapses of comorbid cancers post-transplant. Two patients developed a new malignancy post-transplant, a squamous cell carcinoma of the buccal mucosa treated with local resection and a post-transplant lymphoproliferative disorder treated with rituximab.
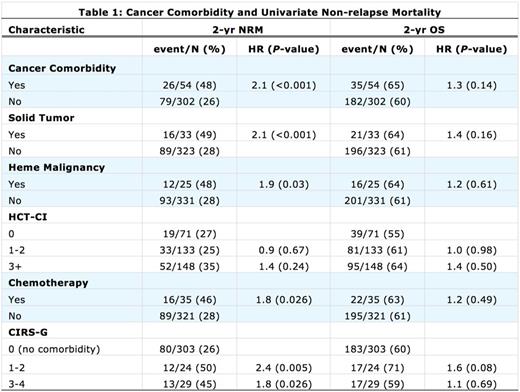
Table 1 shows the association of factors related to cancer comorbidity and unadjusted 2 year NRM. Increased risks were found for cancer comorbidity (HR 2.1), solid tumor (HR 2.1), heme malignancy (HR 1.9), and prior use of chemotherapy to treat comorbid cancer (HR 1.8). When adjusted for age (the only other significant factor for NRM), cancer comorbidity remained statistically significant (HR 1.64, P=0.037); however, categorization in other prognostic groupings were increased but no longer significant including solid tumor (HR 1.58, P=0.1), heme malignancy alone (HR 1.51, P=0.18), or prior chemotherapy for cancer comorbidity (HR 1.47, P=0.17).
Recognizing the study size limitations, 2-year unadjusted overall survival (OS) for cancer comorbidity was not significantly worse (HR 1.33, CI 0.92-1.94) compared to those without prior cancer. Comorbid solid tumor (HR 1.34, CI 0.88-2.22) and heme malignancy (HR 1.15, CI 0.68- 1.95) also did not significantly affect OS.
Conclusions
Hematologic comorbid conditions (7.0%) are nearly as frequent as solid tumor comorbid conditions (9.2%) before allogeneic transplant. Both hematologic and solid cancers likely contribute to high risks of non-relapse mortality, but these are unrelated to relapse of the cancer comorbidity. Additional studies are needed to validate these findings and to better elucidate the mechanisms of increased NRM.
Godley:UpToDate: Honoraria; Onconova, Inc.: Research Funding. Kline:Vasculox: Research Funding; Merck: Honoraria, Research Funding. Liu:BMS: Research Funding; Karyopharm: Research Funding. Odenike:Spectrum: Honoraria, Membership on an entity's Board of Directors or advisory committees; CTI/Baxter: Honoraria, Membership on an entity's Board of Directors or advisory committees; Algeta: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi-Aventis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Research Funding; Suneisis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Stock:ADC Therapeutics: Honoraria; Amgen: Honoraria; Gilead Sciences: Honoraria; Sigma-Tau: Honoraria, Research Funding; Royalties for a chapter in Up to Date: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.