Abstract
INTRODUCTION: Age is no longer a barrier to successful allogeneic hematopoietic cell transplantation (alloHCT). However, reports of alloHCT outcomes in elderly patients aged 65 years (yo) and older are limited. These patients are underrepresented in clinical trials and data usually come from relatively small series and subgroup analyses from the main indications, namely acute leukemia (AL) and myelodysplastic syndromes (MDS). Here, we sought to evaluate the feasibility and general outcomes of the alloHCT procedure in a large cohort of elderly patients reported to the EBMT.
PATIENTS AND METHODS: All consecutive alloHCT in recipients 65 yo and older reported to the EBMT between 2000 and 2014 were included. Data collection and outcome analysis followed EBMT registry and statistical guidelines.
RESULTS: A total of 6046 alloHCT, including 214 second or subsequent procedures, from 270 EBMT centers in 32 countries were identified: median age was 67 (65-84), 4914 were 65-69 yo (Group I) and 1132 ≥70 yo (Group II); 63% were male; 50% had AL, 37% MDS and/or myeloproliferative disorders (MPD), 8% chronic leukemia, 4% lymphoma, 1% bone marrow failure (BMF) and 0.6% plasma cell disorders. They underwent peripheral blood (91%), bone marrow (7%) or cord blood (2%) alloHCT from unrelated (68%), matched (28%) or mismatched related donors (4%). There was female to male sex mismatch in 19% and CMV mismatch in 34% of cases. T cell depletion was used as part of GVHD prophylaxis in 66% of patients.
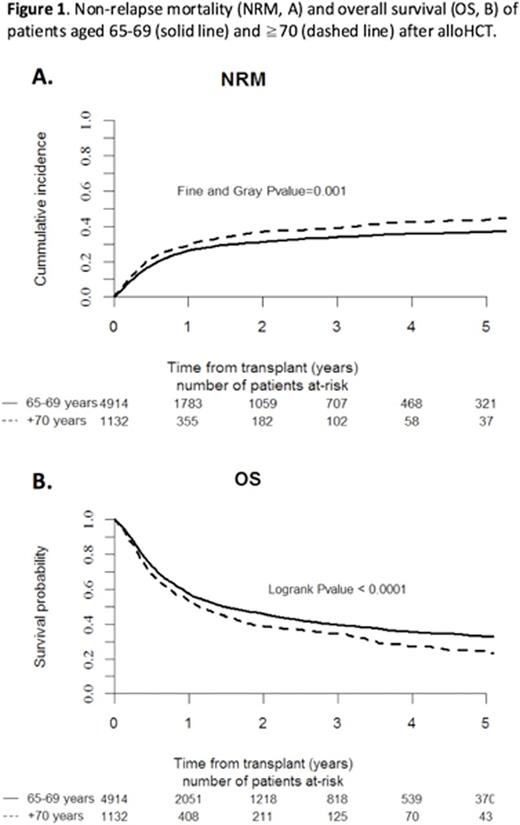
AlloHCT activity in elderly patients increased over the study period from 37 out of 6,413 in 2000 (<1%) to 1057 out of 15,765 in 2014 (6.7%; p <0.001). Neutrophil and platelet engraftment were achieved at median day +16 (IQR 13-20) and +15 (IQR 13-21), respectively, while 6% experienced primary or secondary graft failure. Grade II-IV acute GvHD was observed in 28% of patients, with stage 2-4 involvement of skin in 50%, gut in 28% and liver in 7% of aGvHD cases. Venoocclusive disease was reported in 2%. The incidence of transplant complications did not differ between age groups. Median follow up time for survivors was 13 months (0.2-175). Non-relapse mortality (NRM) was 27% [95%CI 26-28] at 1y and 35% [95%CI 33-36] at 3y and was significantly higher in Group II (29% at 1y and 39% at 3y) than in Group I (26% at 1y and 34% at 3y; p <0.01; Fig. 1A). Multivariate analysis contributing to NRM identified increased age, diagnosis of lymphoma, MDS/MPD or BMF, unrelated or mismatched related donor, sex mismatch female to male and transplantation from CMV negative donor to CMV positive patient, or between both CMV positive as independent prognostic factors (Table 1). Cumulative incidence of relapse was similar in both groups, 24% [95%CI 23-25] at 1y and 32% [95%CI 30-33] at 3y. Cause of death was transplant related in 53% of cases, relapse/progression in 39% and others in 8%, with no difference between age groups. Overall survival was 56.6% [95%CI 55-58] at 1y and 38.6% [95%CI 37-40] at 3y. It was significantly higher in Group I (57.3% at 1y and 39.5% at 3y) than in Group II (53.2% at 1y and 34.7% at 3y; p<0.001, Fig. 1B). Multivariate analysis of factors revealed increased age, diagnosis of lymphoma, unrelated or mismatched related donor, time from diagnosis to alloHCT >12 months, sex mismatch female to male and transplantation from CMV negative donor to CMV positive patient as independent risk factors affecting OS (Table 1). For the subset of alloHCT recipients ≥75 yo, NRM was 26% [95%CI 16-38] at 1y and 34% [95%CI 21-47] at 3y, and OS was 56% [95%CI 45-70] at 1y and 38% [95%CI 27-56] at 3y.
CONCLUSIONS: Improved supportive care and less toxic preparative regimens make alloHCT feasible in elderly patients aged 65 yo and older. This study confirms the feasibility of alloHCT in a large-series of elderly patients, with acceptable NRM and OS at 1 and 3 years, including those with very advanced age of 75 yo and older. In addition, it identifies in the multivariate analysis factors that have an independent impact on the outcome of alloHCT in elderly patients. Undoubtedly, elderly alloHCT recipients in such a registry study are a highly selected population. This nevertheless further endorses the need to assess comorbidity and frailty beyond age in older alloHCT candidates to improve outcomes further. Studies to assess the benefit from alloHCT in elderly patients with particular diseases and indications for alloHCT are warranted.
Dreger:Novartis: Consultancy; Gilead: Speakers Bureau; Gilead: Consultancy; Novartis: Speakers Bureau; Janssen: Consultancy; Roche: Consultancy. Montoto:Roche: Honoraria; Gilead: Research Funding. Niederwieser:Amgen: Speakers Bureau; Novartis Oncology Europe: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.