Abstract
Background: Acute myeloid leukemias (AML) with complex karyotype (CK) are associated with an adverse patient prognosis with current therapies, in particular when they include TP53 mutations. Identification of novel therapeutic strategies is urgently needed for this subgroup of patients.
Methods and aims:We have performed an RNA-Sequencing transcriptomic analysis of 68 CK AML included in the Leucegene cohort comprising 415 primary AML samples and performed correlative targeted chemical screening aiming at the identification of active agents in this subgroup. Cell culture and analysis of mutations, gene expression and chemical screening were performed as previously described (Pabst et al., Nature Methods, 2014; Lavallée et al, Nature Genetics, 2015).
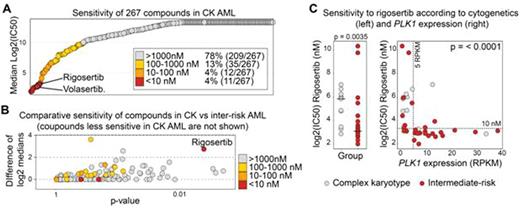
Results:Genes mutated at a frequency greater than 5% in the CK cohort were: TP53 (43/68, 63%), NRAS (9/68, 13%), DNMT3A (8/68, 12%), TET2 and NF1 (7/68 each, 10%), RUNX1 (6/68), FLT3, MLL and PTPN11 (5/68 each) and JAK2 and U2AF1 (4/68 each). Of those, only TP53 (p < 0.0001) and NF1 (p = 0.02) were preferentially associated with CK AML. HMGA2 (High Mobility Group AT-hook 2) was the most differentially expressed gene in CK AML compared to other AML (median: 1.35 vs 0.008 RPKM, q = 2 x 10-14). TP53-mutated samples were in addition characterized by low expression levels of Ectodysplasin A2 Receptor or EDA2R, a known target of TP53. We next tested in dose-response studies 267 compounds enriched in approved anti-cancer drugs on 27 primary CK AML samples and in 11 intermediate-risk karyotype controls. Four percent of these compounds were very active (median IC50 < 10 nM) in CK AML (red dots in Fig A). Surprisingly, this small subset of compounds comprised the only 2 inhibitors of Polo-Like Kinase 1 (PLK1), volasertib (median IC50: 6.6 nM) and rigosertib (median IC50: 8.5 nM). Rigosertib was, in addition, more active in CK AML than in intermediate-risk AML (p = 0.0035) supporting the hypothesis that choice of treatment, rather than genetic anomalies, determine prognosis (Fig. B-C). Most interestingly, expression levels of PLK1 correlated with the measured sensitivity to both rigosertib and volasertib raising the possibility that PLK1 expression determines sensitivity to these inhibitors. PLK1 is more highly expressed in CK AML (p=0.009) and in TP53-mutated AML (p = 0.015) than in control samples. A threshold of 5 RPKM was particularly predictive for drug sensitivity (rigosertib: p < 0.001, Fig C; volasertib: p = 0.037) suggesting that it could become a companion biomarker. Rigosertib and volasertib also potently inhibit PLK2 and PLK3 in cell-free assays, but no association was observed between PLK2/PLK3 expression and response to inhibitors, suggesting that cell lethality was mediated by PLK1 inhibition. PLK1 is a protein kinase involved in cell cycle. Accordingly, expression of this gene in our dataset defines a cluster of very highly correlated genes comprising mostly cell cycle components (e.g. CDC20, CCNB2, CENPA and CTSE1: > 0.90).
Volasertib was recently studied in a phase 2 clinical trial in AML patients in combination with low-dose cytarabine (LDAC) (Döhner et al, Blood 2014). In this analysis, and in line with our data, responses were seen across all genetic groups including in adverse-risk AML (1/14 in LDAC vs 5/14 in LDAC + volasertib).
Conclusion:Our chemo-transcriptomic analysis revealed that CK AML are characterized by high levels of HMGA2 expression and, in addition for the TP53 mutated subset, low levels of EDA2R. Most importantly our results show that CK AML with high expression levels of PLK1 are uniformly and preferentially sensitive to PLK1 inhibitors. Our data thus support the hypothesis that sensitivity to PLK1 inhibitors is associated to PLK1 expression, and that this gene may represent a promising biomarker to predict biologicalresponse to these agents. Our analysis provides for the first time a strong rationale for investigating PLK1 inhibitors in the context of CK and/or TP53 mutated AML in which correlative PLK1 expression analyses are commanded.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.