In this issue of Blood, Wu et al describe a novel dominant negative loss-of-function mutation (BLVRB S111L) in a heme catabolic pathway deregulating reactive oxygen species (ROS) and associated with thrombocytosis.1
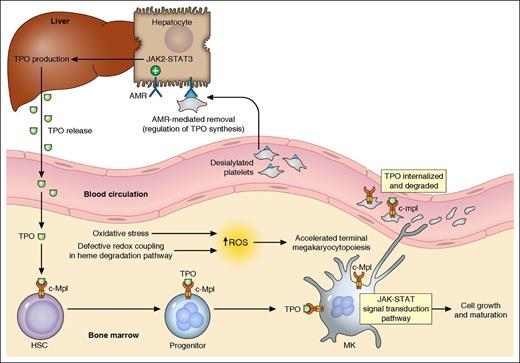
Thrombopoiesis regulation. A simplified summary of the major mechanisms in thrombopoiesis regulation based on the currently available evidence is illustrated. TPO, produced primarily in the liver and released into blood, binds to its receptor c-Mpl (MPL protein, TPO-R) expressed on hematopoietic cells, activates JAK/STAT pathways, and stimulates MK growth/maturation. In the presence of oxidative stress, the defective redox coupling in heme degradation pathway results in increased accumulation of ROS, which leads to accelerated terminal megakaryocytopoiesis and possibly enhanced proplatelet formation. Platelets in circulation, also with c-Mpl expression on the cell surface, can bind to and internalize TPO. The desialylated platelets (eg, from senescence or cold storage) can be removed through the AMR-mediated pathway. The hepatocyte AMRs, upon binding to desialylated platelets, activate downstream JAK2-STAT3 pathways, leading to elevated THPO messenger RNA expression and translation. TPO is then released to stimulate thrombopoiesis. HSC, hematopoietic stem cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
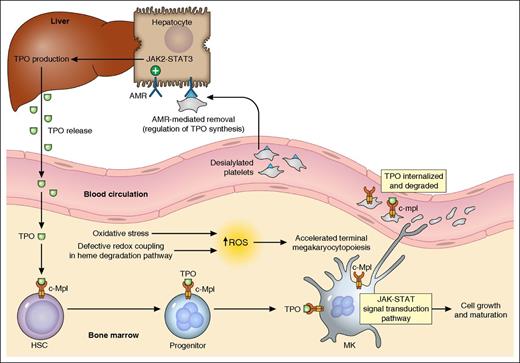
Thrombopoiesis regulation. A simplified summary of the major mechanisms in thrombopoiesis regulation based on the currently available evidence is illustrated. TPO, produced primarily in the liver and released into blood, binds to its receptor c-Mpl (MPL protein, TPO-R) expressed on hematopoietic cells, activates JAK/STAT pathways, and stimulates MK growth/maturation. In the presence of oxidative stress, the defective redox coupling in heme degradation pathway results in increased accumulation of ROS, which leads to accelerated terminal megakaryocytopoiesis and possibly enhanced proplatelet formation. Platelets in circulation, also with c-Mpl expression on the cell surface, can bind to and internalize TPO. The desialylated platelets (eg, from senescence or cold storage) can be removed through the AMR-mediated pathway. The hepatocyte AMRs, upon binding to desialylated platelets, activate downstream JAK2-STAT3 pathways, leading to elevated THPO messenger RNA expression and translation. TPO is then released to stimulate thrombopoiesis. HSC, hematopoietic stem cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
The critical role of platelets in hemostasis and their origin from marrow megakaryocytes (MKs) were first established in the early 20th century. The identification and characterization of the MPL proto-oncogene (MPL, or c-Mpl) followed by thrombopoietin (TPO) are milestones in unraveling the regulation of thrombopoiesis.2,3 TPO, produced primarily in the liver, is the major physiological regulator of platelet production and its level is inversely correlated with platelet count (mass). MPL, the receptor of TPO, is predominantly expressed in hematopoietic tissues with a higher density on MKs.4,5 The delicate maintenance of platelet homeostasis has initially been explained by the “autoregulation” model, which postulates that TPO is produced at a constant rate and cleared by binding to TPO receptors on platelets followed by internalization and degradation.6 However, a growing body of evidence suggests the presence of other regulatory mechanisms in TPO production. Recently, the hepatic Ashwell-Morrell receptor (AMR) was shown to mediate the removal of desialylated platelets and regulate hepatic TPO synthesis, providing a better understanding of the transcriptional regulation of TPO production7,8 (see figure).
Previous work has provided evidence for the existence of additional pathways in the regulation of thrombopoiesis. Choi et al9 reported that the final stages of platelet formation and release appeared to be TPO independent, because withdrawal of TPO from late-stage MK culture did not eliminate proplatelet formation. Ng et al10 reported that mice lacking MPL expression on MKs and platelets, but with preserved MPL expression on stem/progenitor cells, displayed profound megakaryocytosis and thrombocytosis with expansion of progenitor cells.
The study performed by Wu et al opens a new window in understanding the regulation of thrombopoiesis beyond the TPO pathway. Specifically, the authors concluded that increased ROS accumulation as a result of defective redox coupling leads to differential hematopoietic lineage commitment and enhanced thrombopoiesis. RNAseq analysis of highly purified platelets from subjects with essential thrombocythemia (ET) and healthy controls identified 5 single nucleotide variants (SNVs) associated with the ET phenotype. To restrict the SNVs to only the modifiers of platelet production independent of molecular abnormalities associated with myeloproliferative neoplasms (such as JAK2 V617F), the authors genotyped a separate cohort of subjects with reactive thrombocytosis (RT). Only the BLVRB S111L variant retained its significance as a thrombocytosis risk allele in both ET and RT subjects. This variant was subsequently functionally characterized to be a loss-of-function mutation leading to defective redox coupling of the enzyme. These findings established the first physiologically relevant function of BLVRB in the heme degradation pathway, and a possible role of redox signaling in terminal megakaryocytopoiesis (see figure).
The authors present a convincing argument for this mutation uncoupling a redox reaction with generation of increased ROS and an association with thrombocytosis. Although much work remains to be done to fully understand the precise mechanism, this study opens an exciting line of inquiry, which should lead to a better understanding of normal and pathologic thrombopoiesis. This pathway also represents a potential therapeutic target for treatment of pathological thrombocytosis; the utility of intervention in this novel pathway remains to be tested.
Conflict-of-interest disclosure: The authors declare no competing financial interests.