Key Points
Myeloma cells produce ammonium in the presence of glutamine, showing high glutaminase and low glutamine synthetase expression.
Myeloma cells show high expression of glutamine transporters and inhibition of ASCT2 transporter hinders myeloma growth.
Abstract
The importance of glutamine (Gln) metabolism in multiple myeloma (MM) cells and its potential role as a therapeutic target are still unknown, although it has been reported that human myeloma cell lines (HMCLs) are highly sensitive to Gln depletion. In this study, we found that both HMCLs and primary bone marrow (BM) CD138+ cells produced large amounts of ammonium in the presence of Gln. MM patients have lower BM plasma Gln with higher ammonium and glutamate than patients with indolent monoclonal gammopathies. Interestingly, HMCLs expressed glutaminase (GLS1) and were sensitive to its inhibition, whereas they exhibited negligible expression of glutamine synthetase (GS). High GLS1 and low GS expression were also observed in primary CD138+ cells. Gln-free incubation or treatment with the glutaminolytic enzyme l-asparaginase depleted the cell contents of Gln, glutamate, and the anaplerotic substrate 2-oxoglutarate, inhibiting MM cell growth. Consistent with the dependence of MM cells on extracellular Gln, a gene expression profile analysis, on both proprietary and published datasets, showed an increased expression of the Gln transporters SNAT1, ASCT2, and LAT1 by CD138+ cells across the progression of monoclonal gammopathies. Among these transporters, only ASCT2 inhibition in HMCLs caused a marked decrease in Gln uptake and a significant fall in cell growth. Consistently, stable ASCT2 downregulation by a lentiviral approach inhibited HMCL growth in vitro and in a murine model. In conclusion, MM cells strictly depend on extracellular Gln and show features of Gln addiction. Therefore, the inhibition of Gln uptake is a new attractive therapeutic strategy for MM.
Introduction
Multiple myeloma (MM) is characterized by the accumulation of malignant plasma cells (PCs) into the bone marrow (BM).1,2 It is a historical notion that the growth of MM cells was limited by depletion of l-glutamine (Gln)3 and that MM cells may produce an excess of ammonium (NH4+).4,5 Hyperammonemia with or without the related encephalopathy has been reported as a possible rare clinical manifestation in relapsed/refractory MM patients with high mortality rate.6-12 Recently, multivariate analysis based on 1H-NMR spectroscopy analysis of serum samples has shown that a specific metabolic profile characterized MM patients vs healthy controls, including Gln levels significantly lower in the MM group.13 Overall, these data suggest that Gln is highly metabolized in MM cells. To satisfy metabolic requirements of Gln, mammalian cells rely on glutamine synthetase (GS), the enzyme that obtains Gln from glutamate (Glu) and NH4+.14,15 Moreover, a variety of carriers, operate Gln influx, such as the Na+-dependent transporters SNAT1-2 and ASCT2, and the Na+-independent transporter LAT1.16 Gln is a substrate of several enzymes, playing an important role in various processes, such as the synthesis of nucleotides, other amino acids, or glucosamine.17,18 Moreover, through the activity of glutaminases (GLS1 and GLS2), which hydrolyze the amide group obtaining NH4+ and Glu, Gln may fuel the intracellular pool of the Krebs cycle intermediate and anaplerotic substrate 2-oxoglutarate (2-OG, α-ketoglutarate).17,18 Some types of human tumor cells exhibit an high requirement for Gln (“glutamine addiction”)15 and use large amounts of the amino acid as an anaplerotic substrate.19-21 A number of metabolic features have been described in Gln addicted cancer cells, such as high GS expression22 or high expression and/or activity of Gln transporters, such as ASCT2.23 In Gln-addicted cancers, GLS1 inhibition, Gln transporter silencing, inhibitors of Gln uptake or Gln-depleting treatments lead to delayed or arrested tumor growth.24-26 Gln depletion produces a severe metabolic stress and cell death in some types of acute myeloid leukemia (AML)27,28 and in Gln-addicted lymphoid cells.29 Moreover, l-asparaginase (ASNase), the mainstay in the treatment of acute lymphoblastic leukemia (ALL),30,31 hydrolyzes not only asparagine but also Gln, and Gln depletion is the main biochemical mechanism underlying the growth inhibition by ASNase in asparagine synthetase-positive ALL cells.32 Actually, the relationship between NH4+ production and Gln addiction in MM cells, as well as the mechanisms involved therein, are unknown, and were investigated in this study.
Patients, materials, and methods
Patients
A total cohort of 65 patients (30 males and 35 females) with PC disorders were included in the study: 6 patients with monoclonal gammopathy of undetermined significance (MGUS) (median age: 68 years; range: 44-80 years), 12 with smoldering myeloma (SMM) (median age: 68 years; range: 41-83 years), and 46 with active MM (median age: 75 years; range: 43-90 years) including 28 newly diagnosed MM (ND-MM) and 18 relapsed MM (R-MM). The main clinical characteristics of all the patients enrolled in the study are summarized in supplemental Table 1, available on the Blood Web site. Adverse cytogenetic/fluorescence in situ hybridization (FISH) refers to unfavorable immunoglobulin (Ig)H translocations [t(4;14) or t(14;16) or t(14;20)], 17p13 del, and/or 1q21 gain.33,34 A total cohort of nine controls (patients without monoclonal gammopathy with cardiac disease; median age, 58 years; range, 42-72 years) underwent cardiac surgery and were included in the study to obtain normal PCs.
The University of Parma institutional review board (Parma, Italy) approved all the study protocols. All of the patients and controls included in the study gave their written informed consent as laid down in the Declaration of Helsinki.
BM aspirates (5 + 5 mL, treated with EDTA to prevent clotting) were obtained from the iliac crest of MM, SMM, and MGUS patients or from the sternum of controls. BM plasma was collected from 17 MM patients (9 ND-MM and 8 R-MM; ISS: 18% stage I, 35% II and 47% III; adverse FISH: 64%) and 13 patients with indolent monoclonal gammopathies (MGUS and/or SMM) after centrifugation and stored at −20°C until the analysis. Peripheral blood (PB) was obtained from 21 of 46 MM patients (13 ND-MM and 8 R-MM; ISS: 23% I, 28% II, and 48% III; adverse FISH: 29%).
BM cell purification
Both CD138+ PCs and CD138− cell fractions were isolated from BM mononuclear cells (MNCs) by an immunomagnetic method with anti-CD138 mAb conjugated with microbeads (Miltenyi Biotech, Bergisch-Gladbach, Germany) from 38 patients (2 MGUS, 7 SMM, 29 MM, including 17 ND-MM and 12 R-MM) and 4 of 9 controls as previously described.35 FISH analysis was performed on CD138+ PCs as previously described.36
Cell lines, reagents, and cell treatments
Cell lines and reagents were described in supplemental Materials and Methods. RPMI-R5 were established as previously reported.37
Treatment under Gln-free conditions was performed incubating cells in Gln-free RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) dialyzed against a 40× volume of 0.154 M NaCl.
Treatment with Erwinia chrysanthemi ASNase (l-asparagine amido hydrolase, E.C. 3.5.1.1; Jazz Pharmaceuticals Ltd, Oxford, UK) or with the Escherichia coli ASNase (Sigma-Aldrich, Milan, Italy) at concentrations ranging from 0.0001 to 1 U/mL was performed for 48 hours in RPMI-1640 medium plus 10% FBS and Gln at 4 mM. Bortezomib (Janssen-Cilag, Milan, Italy) dose response (concentration range, 1.77-10 nM) was performed in standard growth medium in the presence or in the absence of 0.1 U/mL of E. chrysanthemi ASNase for 48 hours. Moreover, HMCLs were treated with bortezomib (1-16 nM) or E. chrysanthemi ASNase (0.0625-1 U/mL) or the combination of the 2 drugs (16:1) or vehicle for 48 hours.
Cell viability
Cell viability was assessed by adding resazurin (44 μM) to the incubation media.38 After 1 hour, fluorescence was measured at 572 nm with a fluorimeter (EnSpire Multimode Plate Readers; Perkin Elmer, Boston, MA). Synergy between E. chrysanthemi ASNase and Bortezomib was quantified by combination index analysis using CompuSyn software version 1 (http://combosyn.com/).
NH4+ quantification
The quantification of NH4+ was detailed in supplemental Materials and Methods.
Amino acid determination
BM plasma samples were de-proteinized with 10% (wt/vol) 5-sulfosalicylic acid and centrifuged at 12 000g for 10 minutes at 4°C. Supernatants were mixed with 1 volume of LiOH-citrate buffer (pH 2.2), and the intracellular content of amino acids was determined by high performance liquid chromatography analysis with a Biochrom 20 amino acid analyzer (Amersham Pharmacia Biotech; GE Healthcare Europe GmbH, Milan, Italy), as previously described.39
Analysis of the transcriptional profile of glutamine transporters
Real-time polymerase chain reaction analysis
Total cell RNA (1 μg), isolated with GenElute total RNA Miniprep Kit (Sigma-Aldrich), was reverse transcribed as described.25 Gln-related enzyme and transporter mRNA expression was analyzed by real-time polymerase chain reaction (PCR) with the primers reported in supplemental Table 2. Data analysis was made according to the relative standard curve method.42 The mRNA expression of GAC and KGA was evaluated by Taqman gene assay (Life Technologies, Thermo Fisher Scientific, Waltham, MA) by the probes hs01022166_m1 and hs01014019_m1, respectively.
Glutamine uptake
The influx of Gln was measured in RPMI 8226 cells following the method for amino acid transport determination previously described.43
Liquid chromatography tandem mass spectrometry
Cells were seeded in a six-well plate. After 24 hours, growth medium was substituted with fresh medium with or without Gln (4 mM). After 19 hours, cells were washed with ice-cold phosphate-buffered saline, and metabolites were extracted with 1 mL ethanol. Liquid chromatography analyses were carried out with an Agilent HP 1100 pump coupled with a API4000 triple-quadrupole mass spectrometer (AB SCIEX, Framingham, MA) equipped with a TurboIonSpray interface and configured in selected reaction monitoring mode adapting a previously published method.44
Immunoblotting
Immunoblotting analysis was performed as previously described.35 Blots were incubated at 4°C overnight with the following antibodies: anti–β-actin (mouse, monoclonal, 1:5000; Sigma-Aldrich) anti-ASCT2 (rabbit, monoclonal, 1:4000; Cell Signaling Technology, Danvers, MA), anti–β-tubulin (mouse, polyclonal, 1:1000; Santa-Cruz Biotechnology, Santa Cruz, CA), anti–caspase 3 (mouse, monoclonal, 1:167; Active Motif, La Hulpe, Belgium), anti-GAPDH (rabbit, polyclonal, 1:4000; Sigma-Aldrich), anti-GLS1 (rabbit, monoclonal, 1:1000; Abcam, Cambridge, UK), anti-GLS2 (rabbit, polyclonal, 1:1000; Abcam), anti-GS (mouse, monoclonal, 1:1500; BD Transduction Laboratories, Franklin Lakes, NJ), anti-LAT1 (rabbit, polyclonal, 1:1000; Cell Signaling Technology), anti-p70S6K, p-T389 (rabbit, monoclonal, 1:1000; Cell Signaling), and anti-SNAT1 (rabbit, polyclonal, 1:500; Abcam).
Flow cytometry analysis of apoptosis
HMCLs (1 × 106) were treated for 24 hours under the conditions detailed in the legends to Figures 3 and 4. After the experimental treatments, cells were incubated in the dark with anti-human Apo 2.7-PE (clone 2.7A6A3; Beckman Coulter, Milan, Italy) for 30 minutes, washed, and then analyzed using FACSCalibur (Becton Dickinson Biosciences).
Lentiviral infection and ASCT2 knockdown
Lentivirus short hairpin RNA (shRNA) anti-SLC1A5 (Origene, Rockville, MD) was used for ASCT2 stable knockdown in RPMI 8226 and JJN3 cell lines, whereas the scramble lentiviral vector was used as control. Recombinant lentivirus was produced by transient transfection of 293T cells following a standard protocol. HMCLs were infected as previously described,35 and the efficiency of the infection was evaluated as % of positive cells for green fluorescence protein signal by flow cytometry.
In vivo experiments
SCID-NOD mice (Harlan Laboratories, Udine, Italy) were housed under specific pathogen-free conditions. All procedures involving animals were performed in accordance with the National and International current regulations. Eight mice per group were injected subcutaneously with 5 × 106 JJN3 cells stably transfected with anti-SLC1A5 containing plasmid vectors (ΔASCT2) or with the empty vector (Scramble). Tumor growth was monitored at different time points, and 21 days after inoculation mice were euthanized, and autopsies were performed. Tumor mass was measured as previously described.45 Plasmacytomas obtained from tumors removed from mice injected with JJN3 anti-SLC1A5 or JJN3 scramble were either fixed in 10% neutral buffered formalin, embedded in paraffin and stained with hematoxylin and eosin, or lysed for protein extraction and western blot analysis.
Results
Myeloma cells produce NH4+ from glutamine
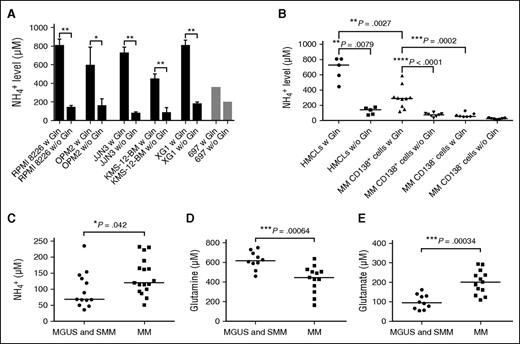
First, we assessed the NH4+ production of several HMCLs (RPMI 8226, OPM2, JJN3, KMS-12-BM, and XG1) and found that all these lines markedly increased NH4+ output in the presence of Gln (Figure 1A). Conversely, the ALL 697 cell line did not (Figure 1A). Primary BM CD138+ PCs from MM patients produced higher NH4+ than the BM CD138− cell fraction from the same patients, as shown for 10 representative MM patients (Mann-Whitney test, P = .0002 in the presence of Gln; Figure 1B). NH4+ production was Gln dependent (P < .0001 for CD138+ fraction; Figure 1B). Comparing NH4+ production between HMCLs and primary CD138+ PCs, we found that the Gln-dependent NH4+ production was higher in HMCLs (P = .0027; Figure 1B). Interestingly, we found that normal PCs obtained from 2 controls produced NH4+ in the presence of Gln at lower levels than MM cells, although the difference did not reach statistical significance (normal PCs: median level, 129.1 μM; primary MM cells, 292.6 μM; P = .09).
MM cells produce ammonium in excess in the presence of Gln. RPMI 8226, OPM2, JJN3, KMS-12-BM, XG1, and 697 cells were seeded at 5 × 105 cells/mL in RPMI-1640 with 10% FBS in the presence (w) or absence (w/o) of Gln (4 mM) and cultured for 16 hours. CMs were collected and immediately analyzed. The CMs of primary BM CD138+ and CD138− fractions of 10 MM patients were also obtained, following the same procedure. Then NH4+ levels were evaluated. (A) Bar graph represents the mean NH4+ plus standard deviation (SD) secreted by cell lines in 2 independent experiments (2-tailed unpaired t test, *P < .05; **P < .01). (B) Plots represent the single values of NH4+ secreted by HMCLs, BM CD138+ MM cells, and BM CD138− cells. (C) Plots represent the single values of BM plasma NH4+ of patients affected by MGUS and SMM (n = 13) and by active MM (n = 17). (D) Gln and (E) glutamate (Glu) in BM plasma of patients with indolent monoclonal gammopathies (MGUS and SMM; n = 10) or active MM (n = 13), evaluated by high performance liquid chromatography. For B-E, lines represent median values for Mann-Whitney test.
MM cells produce ammonium in excess in the presence of Gln. RPMI 8226, OPM2, JJN3, KMS-12-BM, XG1, and 697 cells were seeded at 5 × 105 cells/mL in RPMI-1640 with 10% FBS in the presence (w) or absence (w/o) of Gln (4 mM) and cultured for 16 hours. CMs were collected and immediately analyzed. The CMs of primary BM CD138+ and CD138− fractions of 10 MM patients were also obtained, following the same procedure. Then NH4+ levels were evaluated. (A) Bar graph represents the mean NH4+ plus standard deviation (SD) secreted by cell lines in 2 independent experiments (2-tailed unpaired t test, *P < .05; **P < .01). (B) Plots represent the single values of NH4+ secreted by HMCLs, BM CD138+ MM cells, and BM CD138− cells. (C) Plots represent the single values of BM plasma NH4+ of patients affected by MGUS and SMM (n = 13) and by active MM (n = 17). (D) Gln and (E) glutamate (Glu) in BM plasma of patients with indolent monoclonal gammopathies (MGUS and SMM; n = 10) or active MM (n = 13), evaluated by high performance liquid chromatography. For B-E, lines represent median values for Mann-Whitney test.
Higher NH4+ levels characterized MM patients compared with SMM and MGUS
We screened both PB and BM NH4+ levels in subgroups of MM patients and controls. Among 21 MM out of the total cohort of patients enrolled in the study, we showed that 38% of them had high PB NH4+ levels (standard limits, 10-50 µM) and 14% showed neurological symptoms of encephalopathy without signs of liver dysfunction. In the BM plasma, NH4+ levels were significantly higher in patients with active MM compared with MGUS and SMM (P = .042; Figure 1C). Patients with adverse FISH showed significantly higher BM NH4+ levels than the others (median levels, 163 vs 93.5 μM; P = .006). Interestingly, BM plasma of MM patients had lower Gln and higher Glu compared with that of MGUS and SMM patients (Figure 1D-E), pointing to glutaminase activity as the responsible part of NH4+ production. Amino acid profile in BM plasma is reported in supplemental Table 1.
On the other hand, no significant correlation between the NH4+ levels and % of BM PCs was found (Pearson r2 = 0.1588, P = not significant; data not shown). This observation was further confirmed in another retrospective cohort of 35 patients with monoclonal gammopathy (Pearson r2 = 0.0817, P = not significant; data not shown).
MM cells express high levels of glutaminase but not of GS
The expression of the 2 main enzymes responsible for Gln metabolism, GLS1 and GS, was evaluated in 5 HMCLs and in the ALL 697 cells (Figure 2A). Among the 5 HMCLs, XG1 had the highest expression of GLS1 (total, KGA, GAC), whereas the other 4 cell lines expressed GLS1 mRNAs at levels comparable with those exhibited by ALL cells. Total GLS1 expression and that of the 2 isoforms GAC and KGA were also detected in primary BM CD138+ cells purified from patients with different monoclonal gammopathies, without any significant difference among the groups (Figure 2B). Comparable levels of GLS1 mRNA were found in normal PCs (data not shown). On the contrary, GLS2 is expressed at very low levels in both HMCLs and primary MM cells (supplemental Figure 1). The mRNA of GLUL, the gene that encodes for GS, was much less expressed in the 5 HMCLs than in 697 cells (Figure 2A). HMCLs, with the exception of JJN3 cells, expressed ASNS, the gene for asparagine synthetase, at higher levels than 697 ALL cells (Figure 2A).
MM cells exhibit high expression of GLS1 but not GS. (A) GLS1, GAC, KGA, GLUL, and ASNS expression was analyzed by real-time PCR in HMCLs and 697 cells. Gene expression was normalized to the expression of RPL-15. GAC/KGA mRNA was also reported. Means plus SD of 3 experiments with 2 determinations each are shown. (B) GLS1, GAC, and KGA expression in primary CD138+ cells, purified from 3 MGUS, 5 SMM, 11 ND-MM, and 10 R-MM patients was evaluated with real-time PCR. Lines represent median values. (C) Western blot of GLS1, GS, and ASNS expression by HMCLs and 697 cells. β-tubulin was used for loading control. (D) GS expression in HMCLs and 697 cells incubated for 19 hours in the presence of 4 mM Gln (+) or in the absence of the amino acid (−). (E) GLS1 and GS expression was evaluated by Western blot in CD138+ cells purified from 4 SMM, 7 ND-MM, and 4 R-MM patients; 697 lysate was used as positive control. GAPDH was used for loading control.
MM cells exhibit high expression of GLS1 but not GS. (A) GLS1, GAC, KGA, GLUL, and ASNS expression was analyzed by real-time PCR in HMCLs and 697 cells. Gene expression was normalized to the expression of RPL-15. GAC/KGA mRNA was also reported. Means plus SD of 3 experiments with 2 determinations each are shown. (B) GLS1, GAC, and KGA expression in primary CD138+ cells, purified from 3 MGUS, 5 SMM, 11 ND-MM, and 10 R-MM patients was evaluated with real-time PCR. Lines represent median values. (C) Western blot of GLS1, GS, and ASNS expression by HMCLs and 697 cells. β-tubulin was used for loading control. (D) GS expression in HMCLs and 697 cells incubated for 19 hours in the presence of 4 mM Gln (+) or in the absence of the amino acid (−). (E) GLS1 and GS expression was evaluated by Western blot in CD138+ cells purified from 4 SMM, 7 ND-MM, and 4 R-MM patients; 697 lysate was used as positive control. GAPDH was used for loading control.
All the HMCLs tested showed similar levels of GLS1 protein, with 2 clearly detectable enzyme bands (the higher for native KGA and the lower for the cleaved form GAC; Figure 2C). On the contrary, GS was not detectable in HMCLs but was readily found expressed in 697 cells (Figure 2C). Consistent with mRNA data, ASNS was present in the lysates of all the HMCLs tested at levels comparable (JJN3) or higher than those expressed by ALL 697 cells (Figure 2C). In several cell models, the abundance of GS (protein) is inversely correlated with Gln availability46-48 ; therefore, it is expected that GS will increase when cells are incubated under conditions of Gln shortage. However, even on incubation in the absence of Gln, GS remained undetectable in HMCLs, whereas it was much more expressed in Gln-starved ALL cells compared with Gln–fed counterparts (Figure 2D).
MM cells are dependent on extracellular glutamine and use glutamine for anaplerosis
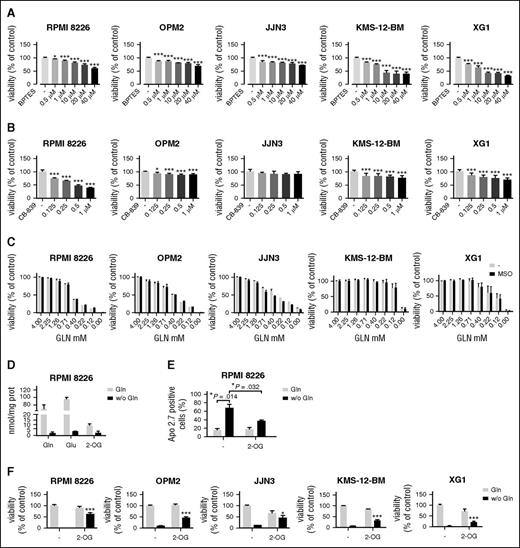
The high expression detected in MM cells prompted us to evaluate the effects of GLS1 inhibition on cell viability. The GLS1 inhibitor bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES)49 significantly lowered cell viability in all the HMCLs tested (Figure 3A), with an effect ranging from 30% for JJN3 to 70% for XG1 cells at the highest dose tested (40 µM). Another GLS1 inhibitor, CB-839 (0.125-1 µM), markedly suppressed viability in RPMI 8226 cultures, whereas it had only small effects in OPM2, KMS-12-BM, and XG1 cells and was ineffective in JJN3 cells (Figure 3B).
MM cells are sensitive to Gln depletion. (A) HMCLs were treated with increasing concentrations of BPTES or vehicle (−). After 48 hours, cell viability was assessed, and data were expressed as % of the value obtained with cells treated with the vehicle. (B) HMCLs were treated with increasing concentrations of CB-839 or vehicle (−). After 48 hours, cell viability was assessed, and data were expressed as % of the value obtained with cells treated with the vehicle. (C) HMCLs were incubated with decreasing concentrations of Gln in the absence or in the presence of the GS inhibitor methionine-sulfoximine (1 mM). After 48 hours, cell viability was assessed, and data were expressed as % of the cell growth observed at 4 mM Gln. (D) Cell contents of Gln, Glu, and 2-OG were measured by liquid chromatography tandem mass spectrometry in RPMI 8226 incubated for 19 hours in the presence (4 mM) or in the absence of Gln. Data were expressed as nmol/mg protein. (E) RPMI 8226 were incubated in the presence (4 mM) or in the absence of Gln with or without dimethyl-2-OG (8 mM). After 24 hours, the expression of the apoptosis marker Apo 2.7 was checked by flow cytometry. (F) MM cells were incubated in the presence (4 mM) or in the absence of Gln with or without dimethyl-2-OG (8 mM). After 48 hours, cell viability was assessed, and data were expressed as % of control (Gln present, 2-OG absent). For panels A-F, data are means ± SD of 3 experiments with 3 determinations each. *P < .05, ***P < .001 vs control.
MM cells are sensitive to Gln depletion. (A) HMCLs were treated with increasing concentrations of BPTES or vehicle (−). After 48 hours, cell viability was assessed, and data were expressed as % of the value obtained with cells treated with the vehicle. (B) HMCLs were treated with increasing concentrations of CB-839 or vehicle (−). After 48 hours, cell viability was assessed, and data were expressed as % of the value obtained with cells treated with the vehicle. (C) HMCLs were incubated with decreasing concentrations of Gln in the absence or in the presence of the GS inhibitor methionine-sulfoximine (1 mM). After 48 hours, cell viability was assessed, and data were expressed as % of the cell growth observed at 4 mM Gln. (D) Cell contents of Gln, Glu, and 2-OG were measured by liquid chromatography tandem mass spectrometry in RPMI 8226 incubated for 19 hours in the presence (4 mM) or in the absence of Gln. Data were expressed as nmol/mg protein. (E) RPMI 8226 were incubated in the presence (4 mM) or in the absence of Gln with or without dimethyl-2-OG (8 mM). After 24 hours, the expression of the apoptosis marker Apo 2.7 was checked by flow cytometry. (F) MM cells were incubated in the presence (4 mM) or in the absence of Gln with or without dimethyl-2-OG (8 mM). After 48 hours, cell viability was assessed, and data were expressed as % of control (Gln present, 2-OG absent). For panels A-F, data are means ± SD of 3 experiments with 3 determinations each. *P < .05, ***P < .001 vs control.
The low levels of GS expression detected in MM cells support the hypothesis that MM Gln metabolism depends on the availability of the extracellular amino acid. Indeed, when incubated in media at decreasing levels of Gln, HMCLs exhibited a progressive loss of viability; in the absence of the amino acid, viability suppression was complete for RPMI 8226, OPM2, and XG1 lines and >90% for JJN3 and KMS-12-BM cells (Figure 3C). Methionine-sulfoximine, an irreversible inhibitor of GS, had no effect, thus excluding a protective role of GS in MM cells (Figure 3C).
To understand the mechanism involved in the loss of viability of MM cells on Gln depletion, we tested whether Gln had an anaplerotic role in MM cells. To this aim, the intracellular levels of Gln, Glu, and 2-OG were measured with mass spectrometry (Figure 3D), demonstrating that Gln-free incubation caused a substantial decrease of the 3 metabolites. A marked depletion of intracellular Glu and 2-OG, along with an increase of intracellular Gln, was also observed on cell treatment with the GLS1 inhibitors BPTES and CB-839 (supplemental Figure 2). As shown in Figure 3E, Gln depletion caused a significant increase in the percentage of apoptotic cells (P = .014), which was partially mitigated in the presence of a membrane-permeant form of 2-OG (Figure 3E). The anaplerotic role of Gln in MM cells and the related protection by 2-OG were confirmed incubating RPMI 8226, OPM2, JJN3, KMS-12-BM, and XG1 cells under conditions of Gln repletion or depletion in the absence or in the presence of 2-OG (Figure 3F). In all the HMCLs, the anaplerotic substrate partially rescued cell viability from the effects of Gln depletion (Figure 3F).
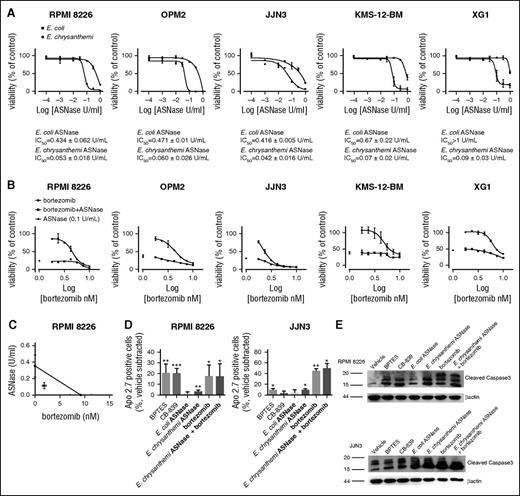
A significant dose–response inhibitory effect on HMCLs proliferation was also observed in the presence of ASNase (Figure 4A). Interestingly, for the 5 HMCLs tested, the IC50 values obtained were ∼10-fold higher for the E. coli than for the E. chrysanthemi enzyme (Figure 4A), which is known to have a glutaminolytic activity ∼10-fold higher compared with the E. coli form. Treatment with ASNase caused a massive decrease of cell Gln, but not of intracellular leucine (supplemental Figure 3), and led to a marked inhibition of mTOR activity (supplemental Figure 4).
MM cells are sensitive to E. chrysanthemi ASNase treatment. (A) HMCLs were treated with increasing doses of l-asparaginase (ASNase) from E. coli or E. chrysanthemi (from 0.0001 to 1 U/mL). After 48 hours, cell viability was assessed, and data were expressed as % of the value obtained with untreated cells. For each HMCL, IC50 for E. coli ASNase and for the E. chrysanthemi enzyme are shown. (B) HMCLs were treated with increasing doses of bortezomib (from 1.77 to 10 nM) or vehicle in the presence or in the absence of E. chrysanthemi ASNase (0.1 U/mL). After 48 hours, cell viability was assessed, and data were expressed as % of the value obtained with the cells treated with vehicle. For panels A and B, data are means ± SD of 3 experiments with 3 determinations each. (C) RPMI 8226 were treated with increasing doses of bortezomib (from 1 to 16 nM), increasing doses of E. chrysanthemi ASNase (from 0.0625 to 1 U/mL), or the combination of the 2 drugs (16:1) or vehicle. After 48 h, cell viability was assessed, and the data were analyzed as % of the value obtained with the cells treated with vehicle. Combination index analysis was then performed using CompuSyn software. Isobologram for ED75 represents means ± SEM of 3 experiments with 5 determinations each. (D-E) RPMI 8226 and JJN3 cells were treated for 24 hours with BPTES (40 µM), CB-839 (1 µM), or ASNase from E. coli (1 U/mL), or ASNase from E. chrysanthemi (0.1 U/mL), or bortezomib (10 nM), or ASNase from E. chrysanthemi (0.1 U/mL) and bortezomib (10 nM), or vehicle. For panel D, cell expression of Apo 2.7 was then evaluated by flow cytometry. The graph shows the mean % plus SD (n = 3) of Apo 2.7 positive cells for each condition after the subtraction of the value obtained in control. For panel E, cells expression of cleaved forms of caspase 3 in HMCLs, evaluated by Western blot. β-Actin was used for loading control. For panels A, B, and D, *P < .05, ***P < .001 vs control.
MM cells are sensitive to E. chrysanthemi ASNase treatment. (A) HMCLs were treated with increasing doses of l-asparaginase (ASNase) from E. coli or E. chrysanthemi (from 0.0001 to 1 U/mL). After 48 hours, cell viability was assessed, and data were expressed as % of the value obtained with untreated cells. For each HMCL, IC50 for E. coli ASNase and for the E. chrysanthemi enzyme are shown. (B) HMCLs were treated with increasing doses of bortezomib (from 1.77 to 10 nM) or vehicle in the presence or in the absence of E. chrysanthemi ASNase (0.1 U/mL). After 48 hours, cell viability was assessed, and data were expressed as % of the value obtained with the cells treated with vehicle. For panels A and B, data are means ± SD of 3 experiments with 3 determinations each. (C) RPMI 8226 were treated with increasing doses of bortezomib (from 1 to 16 nM), increasing doses of E. chrysanthemi ASNase (from 0.0625 to 1 U/mL), or the combination of the 2 drugs (16:1) or vehicle. After 48 h, cell viability was assessed, and the data were analyzed as % of the value obtained with the cells treated with vehicle. Combination index analysis was then performed using CompuSyn software. Isobologram for ED75 represents means ± SEM of 3 experiments with 5 determinations each. (D-E) RPMI 8226 and JJN3 cells were treated for 24 hours with BPTES (40 µM), CB-839 (1 µM), or ASNase from E. coli (1 U/mL), or ASNase from E. chrysanthemi (0.1 U/mL), or bortezomib (10 nM), or ASNase from E. chrysanthemi (0.1 U/mL) and bortezomib (10 nM), or vehicle. For panel D, cell expression of Apo 2.7 was then evaluated by flow cytometry. The graph shows the mean % plus SD (n = 3) of Apo 2.7 positive cells for each condition after the subtraction of the value obtained in control. For panel E, cells expression of cleaved forms of caspase 3 in HMCLs, evaluated by Western blot. β-Actin was used for loading control. For panels A, B, and D, *P < .05, ***P < .001 vs control.
Moreover, Erwinia ASNase effect on HMCLs viability was increased in the presence of bortezomib (Figure 4B). A synergistic effect was obtained for concentrations of bortezomib lower than 9.3 nM and of ASNase lower that 0.35 U/mL, as shown for RPMI 8226 in Figure 4C. Erwinia ASNase significantly reduced cell viability also in bortezomib-resistant RPMI-R5 cells without restoring sensitivity to bortezomib (data not shown).
Finally, the effect on HMCL apoptosis of GLS1 inhibitors, ASNase, and bortezomib was investigated in RPMI 8226 and JJN3 cells. In line with the effects on viability, a significant increase in the percentage of apoptotic cells was found in cells treated with BPTES, CB-839 (only for RPMI 8226 cells), and Erwinia ASNase (alone or in combination with bortezomib) (Figure 4D). The induction of apoptosis in treated cells was also confirmed by the increase of the cleaved caspase 3 forms (Figure 4E).
MM cells and HMCLs overexpressed glutamine transporters
The dramatic effect of extracellular Gln depletion and ASNase on MM cell viability suggests that the transport of Gln from the extracellular compartment is essential for MM cells. Gln uptake is due to several transporters in human cells.23 Therefore, the gene expression profiles of some selected Gln transporters were evaluated in 2 independent PC dyscrasia datasets, obtained either from proprietary or publicly available databases, from the premalignant monoclonal gammopathy up to plasma cell leukemia (PCL) patients, besides healthy donors and HMCLs.
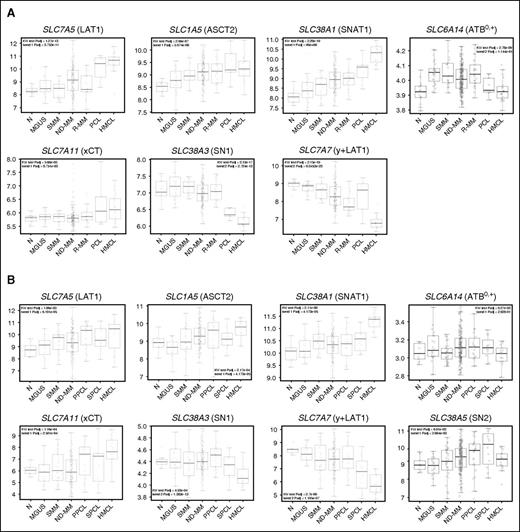
Among the Gln transporters, 3 carriers known to mediate Gln influx, the Na+-independent transporter LAT1 and the Na+-dependent transporters SNAT1 and ASCT2, coded by SLC7A5, SLC38A1, and SLC1A5, respectively, showed a significantly increasing trend in expression levels from normal PCs to HMCLs across the different PC dyscrasias in both the datasets (Figure 5A-B; supplemental Tables 4 and 5). Interestingly, the expression of SNAT1 was also positively correlated with that of MYC (P = 3.965 × 10−12, r = 0.373; P = 1.835 × 10−4, r = 0.22, respectively). ATB0,+, another transporter involved in Gln transport in other cell models, coded by SLC6A14, did not present consistent changes in expression. SN1 and SN2 (coded by, respectively, SLC38A3 and SLC38A5), 2 sodium-dependent, lithium-tolerant systems able to mediate bi-directional fluxes of Gln, exhibited a different behavior. SN1 showed lower expression in secondary PCL (SPCL) and HMCL groups (Figure 5A-B; supplemental Tables 3 and 4), whereas SN2, assessed only in one of the datasets, did not show significant changes in MM compared with the other groups.
Gene expression profiling of the main glutamine transporters by CD138+ cells. (A) Box plot distribution of the expression levels of SLC7A5 (LAT1), SLC1A5 (ASCT2), SLC38A1 (SNAT1), SLC6A14 (ATB0,+), SLC7A11 (xCT), SLC38A3 (SN1), and SLC7A7 (y+LAT1) genes in a 323-sample dataset, including 18 healthy donors (N), 28 MGUS, 19 SMM, 200 ND-MM, 26 R-MM, 9 PCL patients, together with 23 HMCLs. This 323-sample dataset was generated using GSE13591, GSE6205, GSE6477, and GSE6691 dataset, profiled on GeneChip Human Genome U133A arrays. (B) Box plot distribution of the expression levels of the same Gln transporter genes shown in panel A plus SLC38A5 (SN2) in a 283-sample dataset, comprising 9 N, 20 MGUS, 33 SMM, 170 ND-MM, 24 primary PCL (PPCL), and 9 SPCL cases and also including 18 HMCLs. This 283-sample dataset was obtained using GSE66293 and GSE47552 dataset, analyzed on GeneChip Human Gene 1.0 ST array. For panels A and B, the significance of Kruskal-Wallis and Jonckheere-Terpstra tests was indicated.
Gene expression profiling of the main glutamine transporters by CD138+ cells. (A) Box plot distribution of the expression levels of SLC7A5 (LAT1), SLC1A5 (ASCT2), SLC38A1 (SNAT1), SLC6A14 (ATB0,+), SLC7A11 (xCT), SLC38A3 (SN1), and SLC7A7 (y+LAT1) genes in a 323-sample dataset, including 18 healthy donors (N), 28 MGUS, 19 SMM, 200 ND-MM, 26 R-MM, 9 PCL patients, together with 23 HMCLs. This 323-sample dataset was generated using GSE13591, GSE6205, GSE6477, and GSE6691 dataset, profiled on GeneChip Human Genome U133A arrays. (B) Box plot distribution of the expression levels of the same Gln transporter genes shown in panel A plus SLC38A5 (SN2) in a 283-sample dataset, comprising 9 N, 20 MGUS, 33 SMM, 170 ND-MM, 24 primary PCL (PPCL), and 9 SPCL cases and also including 18 HMCLs. This 283-sample dataset was obtained using GSE66293 and GSE47552 dataset, analyzed on GeneChip Human Gene 1.0 ST array. For panels A and B, the significance of Kruskal-Wallis and Jonckheere-Terpstra tests was indicated.
Two other amino acid transporters, not directly responsible for Gln uptake, also showed significant changes in both the datasets. In particular, xCT (SLC7A11) increased from normal PCs to HMCLs. Conversely, y+LAT1 (SLC7A7), the low expression of which has been recently described as associated to favorable outcome,50 had an opposite trend (Figure 5A-B; supplemental Tables 3 and 4).
MM cells mainly depend on ASCT2 for glutamine transport
On the basis of the gene expression profiling data, we focused our attention on the transporters potentially involved in Gln transport in HMCLs. Preliminarily, we excluded that SN1 or SN2 played a significant role in Gln influx in HMCLs. Although SN2 seems more expressed than SN1 in HMCLs, the overall contribution of the 2 systems to Gln influx was at best marginal, because lithium did not appreciably stimulate the uptake of Gln in the absence of sodium (supplemental Figure 5). Also ATB0,+ did not seem to contribute to Gln uptake in HMCLs, because the expression of SLC6A14 was exceedingly low compared with human airway Calu-3 cells used as a positive control, and, consistently, the preferential substrate D-Ser did not affect significantly Gln influx in MM cells (supplemental Figure 5).
On the contrary, SNAT1, ASCT2, and LAT1 were clearly expressed in all the HMCLs tested at both mRNA (Figure 6A) and protein levels (Figure 6B). The contribution of these transporters to Gln uptake was estimated in RPMI 8226 cells assessing the effects of amino acid analogs (MeAIB, GPNA, and BCH), which work as preferential inhibitors of Gln uptake through, respectively, SNAT1, ASCT2, and LAT1 (Figure 6C). Only GPNA caused a marked inhibition of Gln uptake (−60%; Figure 6C). The possibility that ASCT2 inhibition could be due to the products of GPNA hydrolysis glutamate and p-nitrophenol has been excluded, demonstrating that, at concentrations markedly larger than those expected during the assay period, the 2 compounds are without significant effects on Gln uptake (supplemental Figure 6). ASCT2 expression was also examined in lysates from CD138+ cells of SMM and MM patients (Figure 6D). The transporter was detected in all the samples, and a trend of higher transporter expression was found in R-MM (Figure 6D). SLC1A5 mRNA was also expressed by normal PCs at similar level of CD138+ cells obtained from patients with monoclonal gammopathies (data not shown).
ASCT2 is the major glutamine transporter in MM cells. (A) SLC38A1, SLC1A5, and SLC7A5 gene expression in MM cells, incubated in standard growth medium ([Gln] = 4 mM), were analyzed through real-time PCR. Transporter expression in the human hepatocellular carcinoma cell line HepG2 was used as a positive control. Gene expression was normalized to the expression of RPL-15. Means ± SD of 3 experiments, with 2 determinations each, are shown. (B) SNAT1, ASCT2, and LAT1 expression in HMCLs, incubated in standard growth medium, was analyzed by western blot. HepG2 cells were used as a positive control. β-Tubulin was used for loading control. (C) 1-Min uptake of l-[3,4-3H(N)] Gln (0.6 mM, 20 μCi/mL; Amersham) by RPMI 8226 was performed in serum-free culture medium in the absence (−) or in the presence of the transport inhibitors α-(methylamino)isobutyric acid (MeAIB, 20 mM), l-γ-glutamyl-p-nitroanilide (GPNA, 3 mM), or 2-amino-2-norbornanecarboxylic acid (BCH, 20 mM). Means ± SD of 3 experiments, with 5 independent determinations each, are shown. ***P<.001 vs control. (D) ASCT2 expression was investigated in lysates of the CD138+ population isolated from monoclonal gammopathies patients. The same membrane shown in Figure 2E was blotted with anti-ASCT2 antibody.
ASCT2 is the major glutamine transporter in MM cells. (A) SLC38A1, SLC1A5, and SLC7A5 gene expression in MM cells, incubated in standard growth medium ([Gln] = 4 mM), were analyzed through real-time PCR. Transporter expression in the human hepatocellular carcinoma cell line HepG2 was used as a positive control. Gene expression was normalized to the expression of RPL-15. Means ± SD of 3 experiments, with 2 determinations each, are shown. (B) SNAT1, ASCT2, and LAT1 expression in HMCLs, incubated in standard growth medium, was analyzed by western blot. HepG2 cells were used as a positive control. β-Tubulin was used for loading control. (C) 1-Min uptake of l-[3,4-3H(N)] Gln (0.6 mM, 20 μCi/mL; Amersham) by RPMI 8226 was performed in serum-free culture medium in the absence (−) or in the presence of the transport inhibitors α-(methylamino)isobutyric acid (MeAIB, 20 mM), l-γ-glutamyl-p-nitroanilide (GPNA, 3 mM), or 2-amino-2-norbornanecarboxylic acid (BCH, 20 mM). Means ± SD of 3 experiments, with 5 independent determinations each, are shown. ***P<.001 vs control. (D) ASCT2 expression was investigated in lysates of the CD138+ population isolated from monoclonal gammopathies patients. The same membrane shown in Figure 2E was blotted with anti-ASCT2 antibody.
Activity of ASCT2 is needed for MM growth
To evaluate the effects of Gln transporters on MM cell growth, the transport inhibitors were added to the culture medium of MM cells at the same concentrations used for the inhibition of Gln uptake (Figure 7A). GPNA had the largest growth inhibitory effect, roughly corresponding to a 70% loss of cell viability compared with untreated controls. Also BCH produced a marked decrease in MM cell viability (>50%), whereas the SNAT1 inhibitor MeAIB produced only a minimal effect.
ASCT2 silencing by lentiviral vector impairs MM cell growth in vitro and in vivo. (A) RPMI 8226 cells were incubated in growth medium ([Gln] = 0.6 mM) in the absence (control) or in the presence of MeAIB (20 mM), GPNA (3 mM), or BCH (20 mM). After 72 hours, cell viability was assessed, and results were expressed as % of control. Data represent means ± SD of 3 experiments with 3 determinations each. **P < .01, ***P < .001 vs control as assessed with a 2-tailed Student t test for unpaired data. (B-C) ASCT2 expression in scramble and (B) ΔASCT2 RPMI 8226 and (C) JJN3 cells. Gene expression was evaluated with qRT-PCR and normalized to the expression of RPL-15. ASCT2 protein expression was evaluated with western blot and β-tubulin was used for loading control. (D) 1-Min uptake of Gln (0.6 mM) was performed in scramble and ΔASCT2 RPMI 8226 cells in culture medium in the absence (−) or in the presence of GPNA (3 mM). ***P < .001 vs control. $$$P<.001 vs scramble, as assessed with a 2-tailed Student t test for unpaired data. (E) Scramble and ΔASCT2 RPMI 8226 and JJN3 cells, both at 5 × 105 cells/mL, were grown for 72 hours in medium at 0.6 mM Gln. Cell growth was monitored at the indicated times with the resazurin assay. Data represent means ± SD of 2 experiments with 3 determinations each. SD are shown when greater than the size of the point. (F-H) Two groups of 8 SCID/NOD animals each were injected subcutaneously with 5 × 106 JJN3 cells transfected with a lentiviral vector containing shRNA against ASCT2 (ΔASCT2) or with the control vector (Scramble). Twenty-one days after cell inoculation, mice were killed, and tumors were removed and measured as described in “Patients, materials, and methods.” (F) The box plot graph represents the median volume of the masses (P calculated by Mann-Whitney test). (G) Representative picture of tumors obtained from mice injected with JJN3 Scramble and ΔASCT2 cells stained with hematoxylin-eosin (original magnification, 1×). (H) ASCT2 expression was assessed in plasmacytomas removed after animal death. β-Tubulin was used for loading control.
ASCT2 silencing by lentiviral vector impairs MM cell growth in vitro and in vivo. (A) RPMI 8226 cells were incubated in growth medium ([Gln] = 0.6 mM) in the absence (control) or in the presence of MeAIB (20 mM), GPNA (3 mM), or BCH (20 mM). After 72 hours, cell viability was assessed, and results were expressed as % of control. Data represent means ± SD of 3 experiments with 3 determinations each. **P < .01, ***P < .001 vs control as assessed with a 2-tailed Student t test for unpaired data. (B-C) ASCT2 expression in scramble and (B) ΔASCT2 RPMI 8226 and (C) JJN3 cells. Gene expression was evaluated with qRT-PCR and normalized to the expression of RPL-15. ASCT2 protein expression was evaluated with western blot and β-tubulin was used for loading control. (D) 1-Min uptake of Gln (0.6 mM) was performed in scramble and ΔASCT2 RPMI 8226 cells in culture medium in the absence (−) or in the presence of GPNA (3 mM). ***P < .001 vs control. $$$P<.001 vs scramble, as assessed with a 2-tailed Student t test for unpaired data. (E) Scramble and ΔASCT2 RPMI 8226 and JJN3 cells, both at 5 × 105 cells/mL, were grown for 72 hours in medium at 0.6 mM Gln. Cell growth was monitored at the indicated times with the resazurin assay. Data represent means ± SD of 2 experiments with 3 determinations each. SD are shown when greater than the size of the point. (F-H) Two groups of 8 SCID/NOD animals each were injected subcutaneously with 5 × 106 JJN3 cells transfected with a lentiviral vector containing shRNA against ASCT2 (ΔASCT2) or with the control vector (Scramble). Twenty-one days after cell inoculation, mice were killed, and tumors were removed and measured as described in “Patients, materials, and methods.” (F) The box plot graph represents the median volume of the masses (P calculated by Mann-Whitney test). (G) Representative picture of tumors obtained from mice injected with JJN3 Scramble and ΔASCT2 cells stained with hematoxylin-eosin (original magnification, 1×). (H) ASCT2 expression was assessed in plasmacytomas removed after animal death. β-Tubulin was used for loading control.
To assess the effects of ASCT2 silencing on MM cell viability, a lentiviral vector was used to transfect RPMI 8226 and JJN3 cells with an anti-SLC1A5 shRNA. A marked repression (>80%) of the transporter expression was obtained at both mRNA and protein level in silenced cells compared with cells transfected with the scramble control (Figure 7B-C). Gln influx was substantially lower in ASCT2-silenced than in scramble-transfected cells (Figure 7D). Moreover, the portion of Gln transport inhibited by GPNA was markedly smaller in ASCT2-silenced than in control cells, indicating that the different transport rates were effectively due to ASCT2 silencing. Compared with the scramble-transfected control, both ASCT2-silenced (ΔASCT2) RPMI 8226 and JJN3 cells exhibited a lower growth (2-tailed t test, RPMI 8226 ΔASCT2 vs RPMI 8226 Scramble, P = 3.9 × 10−9; JJN3 ΔASCT2 vs JJN3 Scramble, P = .0002; Figure 7E). Either by flow cytometry (Apo 2.7 staining) or western blotting (Caspase 3 activation), we did not obtain clear cut signs of cell death/apoptosis induced by ASCT2 silencing (data not shown). Although statistical significance was not reached, ASCT2-silenced cells exhibited a trend to consume less Gln than control cells (supplemental Figure 7).
Stable ASCT2 silencing inhibited MM growth in vivo
Finally, to confirm in vivo our in vitro evidence, we investigated whether the silencing of ASCT2 influences MM cell tumor growth in a murine xenograft model. To this purpose, JJN3 cells, transfected with the anti-ASCT2 shRNA (ΔASCT2) or with the scramble control (Scramble), were injected subcutaneously into SCID-NOD animals. As shown in Figure 7F, mice inoculated with the ASCT2-silenced cells developed significantly smaller tumors than animals injected with the scramble-transfected cells. A significant reduction of the tumor size was confirmed after plasmacytoma explant and hematoxylin-eosin staining, as shown for 2 representative mice (Figure 7G). At death, the expression of ASCT2 was markedly lower in ΔASCT2 than in scramble-transfected tumors, as confirmed by western blot (Figure 7H).
Discussion
Hyperammonemia has been reported as a possible feature of MM patients.7-12 Single cases have been described in the literature and retrospective screening of the database of MM patients. Otsuki et al found that 60% of 20 patients that died from MM had high serum NH4+ levels, although data on liver function and the presence of encephalopathy were not reported.4 On the other hand, Matsuzaki et al reported that 7% of 85 patients had hyperammonemia associated with neurologic signs.7 Similarly, the Arkansas group found 3.8% of 209 patients with hyperammonemia and encephalopathy, without liver dysfunction.10 In our prospective cohort of MM patients, we show that ∼38% of 21 patients analyzed, without liver dysfunction, had high peripheral NH4+ levels, but only 14% had signs of encephalopathy. Despite the differences observed in the prevalence of hyperammonemia, attributable to the different series of patients analyzed or, as recently reported, to the high technical variability in testing serum NH4+ levels,51 overall, these observations support the hypothesis that MM cells may produce NH4+. In fact, although excess NH4+ production by HMCLs has been demonstrated in vitro,4,5 the mechanisms involved and the possible relationship with the dependence of their growth on Gln3 have not been investigated.
In this study, first, we show that not only HMCLs but also CD138+ PCs from MM patients produce NH4+ from Gln. Indeed, analyzing BM plasma NH4+ levels in different cohorts of patients with monoclonal gammopathies, we show that MM patients had significant higher levels than those with SMM and MGUS, without a significant relationship with the number of BM PCs. Higher NH4+ was associated with higher Glu and lower Gln levels, which indicates that neoplastic PCs exert active glutaminolysis in vivo. Accordingly, expression of GLS1 is consistently present in HMCLs and detected in primary CD138+ cells from almost all MM patients. Second, we demonstrate that Gln represents an absolute nutritional requirement for neoplastic PCs and that these cells, lacking a detectable expression of GS, exclusively rely on the uptake of extracellular Gln to satisfy their needing for the amino acid. This is consistent with high sensitivity of HMCLs to the depletion of extracellular Gln and to the silencing/inhibition of the Gln transporter ASCT2.
Although the sensitivity shown by each HMCL toward BPTES and CB389 is different, glutaminase inhibitors also hinder HMCL cell growth and induce apoptosis, suggesting that glutaminolysis has an important role in MM cell metabolism. Through glutaminolysis, Gln replenishes the intracellular pool of Glu, which, through transaminases or Glu dehydrogenase, provides the anaplerotic Krebs cycle intermediate 2-OG. As shown in several human cancer models,18,23,52,53 Gln-dependent anaplerosis is one of the mechanisms likely underlying Gln addiction, which implies the needing for large amounts of the amino acid. The anaplerotic role of Gln in MM cells and the Gln addiction of this cancer model are confirmed by the fall in 2-OG levels observed in Gln-depleted cells and by the rescue of Gln-depleted myeloma cells observed on medium supplementation with the membrane-permeant analog dimethyl-2-OG. However, viability rescue from 2-OG is only partial, indicating that, besides anaplerosis, Gln plays other prosurvival roles in MM cells. Indeed, whereas 2-OG easily supplies the intracellular Glu pool, the conversion of Glu to Gln is still prevented in MM cells by the absence of GS. Consequently, all the pathways that exhibit an absolute requirement for Gln will be severely hampered if a GS-negative MM cell is incubated under Gln-free conditions. Thus, human MM cells present a thus far unknown association between signs of Gln addiction and lack of expression of GS, 2 features that synergistically increase the dependence of MM cells on extracellular Gln.
Consistently, HMCLs are more sensitive to E. chrysanthemi ASNase than to the E. coli enzyme. Although the 2 enzymes have comparable asparaginolytic activities, they differ as far as Gln hydrolysis is concerned, with the Erwinia enzyme endowed with a 10-fold higher activity. Interestingly, the 5 HMCLs tested exhibited comparable IC50 values for Erwinia ASNase, although they express asparagine synthetase at different levels. Moreover, ASNase caused a massive depletion of intracellular Gln but not of leucine (supplemental Figure 3) and markedly inhibited mTOR activity (supplemental Figure 4). However, rapamycin had much smaller effects on cell viability than ASNase (supplemental Figure 4). Collectively, these data suggest that the hydrolysis of extracellular Gln, followed by the depletion of the intracellular Gln pool, is the prevalent mechanism of the antimyeloma activity exhibited by ASNase.
These considerations highlight the critical importance that Gln transport assumes for MM cells, as suggested by the changes in gene expression across the progression of human PC dyscrasias. Many transporters are potentially involved in Gln transport.16,54 However, the contribution of ATB0,+ (SLC6A14), SN1 (SLC38A3), and SN2 (SLC38A5), known to interact with Gln in other cell models,16,54 did not appear important (supplemental Figure 5). Three other transporters, LAT1, SNAT1, and ASCT2, showed increased expression during MM progression, suggesting that the affirmation of the neoplastic clone may require a growing supply of the amino acid through their operation. The relative contribution of each transporter to the uptake depends on several factors, the most important of which is the affinity toward Gln.16 For this reason, the discrimination of transporter contribution to Gln uptake in MM cells has been performed at a concentration of Gln comparable to that present in human plasma. Under these conditions, ASCT2, estimated from the portion of uptake inhibited by GPNA, accounts for most of Gln influx, with SNAT1 and LAT1 restricted to minor roles. Consistently, GPNA suppressed MM cell viability. However, growth inhibition by the LAT1 inhibitor BCH was much larger than its effect on Gln uptake. This apparent anomaly may be explained considering that LAT1 also mediates the influx of many essential amino acids.
After showing that also ASCT2 silencing in vitro had significant inhibitory effects on MM cell growth in 2 different HMCLs, we confirmed these results with a xenograft model, although tumor growth inhibition was only partial. This result, comparable to data obtained with similar approaches in other human cancer models,15,23,27 suggests that, when faced with scarce Gln fluxes from the extracellular compartment, MM cells can adopt escaping strategies based on the operation of other transporters. A good candidate could be the SNAT1 transporter, which, although found overexpressed in the genome-wide expression analysis during MM progression and nicely expressed in HMCLs, seems to account for a very minor portion of Gln uptake under control (Gln repleted) conditions. The SLC7A11 gene has been also found overexpressed in MM cells. This gene encodes for xCT, an important transporter needed for exchanging intracellular Glu and extracellular cystine, which are involved in the cell response to oxidative stress.55
Finally, our data indicate that blocking Gln uptake could be, possibly in association with other approaches, a suitable target to inhibit MM cell growth as also reported for other hematologic malignancies.23,24,27 In line with this hypothesis, we show that bortezomib increased the cytotoxic effect of E. chrysanthemi ASNase as previously reported for acute leukemia cells.56
Others recently showed that CB-839 blocks MM growth and synergized with pomalidomide in a preclinical model,57 and a phase 1 trial with CB-839 in patients with R-MM is currently under investigation.58 Moreover, it has been recently reported that Gln withdrawal enhanced MM cell sensitivity to BH3 mimetics venetoclax (ABT-199), a new antimyeloma drug currently under investigation,59 and that ritonavir increases the Gln reliance of MM cells.60 Overall, these data and our results suggest that Gln addiction and uptake could be a potential new therapeutic strategy in MM patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dirce Gennari for technical support.
This work was supported in part by a grant from the Associazione Italiana per la Ricerca sul Cancro (AIRC) Investigator Grant (IG) 2014 15531 (N.G.), AIRC IG 13018 (I.A.), and a fellowship Fondazione Italiana per la Ricerca sul Cancro 18152 (M.B.).
Authorship
Contribution: M.B. and M.C. performed all the in vitro experiments, supported by M.G.B., M.A., A.B., D.T., F.C., and V.M; R.A. performed the liquid chromatography tandem mass spectrometry analysis; F.A., O.B., and N.G. designed the study; M.B., M.C., O.B., and N.G. analyzed data and wrote the manuscript; F.A., F.N., and N.G. provide clinical data and patients; I.A. performed the in vivo studies; R.V. generated the flow cytometry data; G.D. designed the lentiviral approach; K.T. and L.A. generated the gene expression profile analyses; G.M. and A.C. were responsible for ammonium quantification; C.M. and E.M. performed the histologic analysis; M.B., M.C., F.A., P.S., O.B., and N.G. were involved in the interpretation of the results; and V.D. and F.A. read, provided comments, and approved the final version of the manuscript.
Conflict-of-interest disclosure: The research fellowship of M.C. is partially supported by a grant from Jazz Pharmaceuticals to O.B. The remaining authors declare no competing financial interests.
Correspondence: Nicola Giuliani, Myeloma Unit, Department of Clinical and Experimental Medicine, University of Parma, Via Gramsci 14, 43126 Parma, Italy; e-mail: nicola.giuliani@unipr.it; or Ovidio Bussolati, Unit of General Pathology, Department of Biomedical, Biotechnological and Translational Sciences, University of Parma, Via Volturno 39, 43125 Parma, Italy; e-mail: ovidio.bussolati@unipr.it.
References
Author notes
M.B., M.C., and F.A. contributed equally to this work.


![Figure 6. ASCT2 is the major glutamine transporter in MM cells. (A) SLC38A1, SLC1A5, and SLC7A5 gene expression in MM cells, incubated in standard growth medium ([Gln] = 4 mM), were analyzed through real-time PCR. Transporter expression in the human hepatocellular carcinoma cell line HepG2 was used as a positive control. Gene expression was normalized to the expression of RPL-15. Means ± SD of 3 experiments, with 2 determinations each, are shown. (B) SNAT1, ASCT2, and LAT1 expression in HMCLs, incubated in standard growth medium, was analyzed by western blot. HepG2 cells were used as a positive control. β-Tubulin was used for loading control. (C) 1-Min uptake of l-[3,4-3H(N)] Gln (0.6 mM, 20 μCi/mL; Amersham) by RPMI 8226 was performed in serum-free culture medium in the absence (−) or in the presence of the transport inhibitors α-(methylamino)isobutyric acid (MeAIB, 20 mM), l-γ-glutamyl-p-nitroanilide (GPNA, 3 mM), or 2-amino-2-norbornanecarboxylic acid (BCH, 20 mM). Means ± SD of 3 experiments, with 5 independent determinations each, are shown. ***P<.001 vs control. (D) ASCT2 expression was investigated in lysates of the CD138+ population isolated from monoclonal gammopathies patients. The same membrane shown in Figure 2E was blotted with anti-ASCT2 antibody.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/5/10.1182_blood-2016-01-690743/4/m_667f6.jpeg?Expires=1765270697&Signature=Yymbn6CEjO-VC0sTO68aE6B0cTMEx1f2wX1ZMleyss8q~Ujfw~eRWqbuqd-tpp33wn7V-K8d49prv0XC41tITTgAFI5bojtk-jjwlPCeIsL-dYaPKfFQks~DYpimLe9QCjwbMNqD3rjgXr9S1TsysXu6GLvhWh1GnAoHBCBG2K2W4fcBfJE-CciIniHTkws2VxFOB~oJprNJ-CvZXg~0yvB8Sj1NrXWBuf~6jVPV0VumwsYjZqvmDkJZ759sFNiyooyrfP3Lm9llhrvkWLrO4Gox9634x-LUrAx4Vzuvz30DvYZBEKba8utJyiEVIWYdfXF4LijSGYoY-wl7pvHRyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. ASCT2 silencing by lentiviral vector impairs MM cell growth in vitro and in vivo. (A) RPMI 8226 cells were incubated in growth medium ([Gln] = 0.6 mM) in the absence (control) or in the presence of MeAIB (20 mM), GPNA (3 mM), or BCH (20 mM). After 72 hours, cell viability was assessed, and results were expressed as % of control. Data represent means ± SD of 3 experiments with 3 determinations each. **P < .01, ***P < .001 vs control as assessed with a 2-tailed Student t test for unpaired data. (B-C) ASCT2 expression in scramble and (B) ΔASCT2 RPMI 8226 and (C) JJN3 cells. Gene expression was evaluated with qRT-PCR and normalized to the expression of RPL-15. ASCT2 protein expression was evaluated with western blot and β-tubulin was used for loading control. (D) 1-Min uptake of Gln (0.6 mM) was performed in scramble and ΔASCT2 RPMI 8226 cells in culture medium in the absence (−) or in the presence of GPNA (3 mM). ***P < .001 vs control. $$$P<.001 vs scramble, as assessed with a 2-tailed Student t test for unpaired data. (E) Scramble and ΔASCT2 RPMI 8226 and JJN3 cells, both at 5 × 105 cells/mL, were grown for 72 hours in medium at 0.6 mM Gln. Cell growth was monitored at the indicated times with the resazurin assay. Data represent means ± SD of 2 experiments with 3 determinations each. SD are shown when greater than the size of the point. (F-H) Two groups of 8 SCID/NOD animals each were injected subcutaneously with 5 × 106 JJN3 cells transfected with a lentiviral vector containing shRNA against ASCT2 (ΔASCT2) or with the control vector (Scramble). Twenty-one days after cell inoculation, mice were killed, and tumors were removed and measured as described in “Patients, materials, and methods.” (F) The box plot graph represents the median volume of the masses (P calculated by Mann-Whitney test). (G) Representative picture of tumors obtained from mice injected with JJN3 Scramble and ΔASCT2 cells stained with hematoxylin-eosin (original magnification, 1×). (H) ASCT2 expression was assessed in plasmacytomas removed after animal death. β-Tubulin was used for loading control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/5/10.1182_blood-2016-01-690743/4/m_667f7.jpeg?Expires=1765270697&Signature=P91YtcXv1fsuM0fk9QnuqWfGqOt-8HqmK3nLQP-~vlTe50zht1qmH9rdKZX4hgByZlm4qeD9ZEGDmyxNsYM9Q4ZbrD2Y-0iwf0P79QWxrkgEPDNRwyAgj~t6-ww-fny8dpu1rzTU0liw0mvqMweYZAtrxeIiowDTEBMXzWOPgIG3nmuSuhO2lH~Y56n8kMlJePePniK1n3OVxZNRQ3dkJ4LDXckEOCGVxt01QUEcmD5TKxhnAllGh-jcQrsCqRvcR~gRHq05HCfiLPpOT~Uccr47Gp06WtkZ1znQgfXlbriroKymIfZViIZhJlBqCWnw3uoJlIQsSjH7F2ksaczLMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
