Key Points
ATRA promotes ETosis leading to procoagulant promyelocytic extracellular chromatin.
Extracellular chromatin fosters excess thrombin production and fibrin deposition, increases plasmin, and causes endothelium damage.
Abstract
Despite routine treatment of unselected acute promyelocytic leukemia (APL) with all-trans-retinoic acid (ATRA), early death because of hemorrhage remains unacceptably common, and the mechanism underlying this complication remains elusive. We have recently demonstrated that APL cells undergo a novel cell death program, termed ETosis, which involves release of extracellular chromatin. However, the role of promyelocytic extracellular chromatin in APL-associated coagulation remains unclear. Our objectives were to identify the novel role of ATRA-promoted extracellular chromatin in inducing a hypercoagulable and hyperfibrinolytic state in APL and to evaluate its interaction with fibrin and endothelial cells (ECs). Results from a series of coagulation assays have shown that promyelocytic extracellular chromatin increases thrombin and plasmin generation, causes a shortening of plasma clotting time of APL cells, and increases fibrin formation. DNase I but not anti-tissue factor antibody could inhibit these effects. Immunofluorescence staining showed that promyelocytic extracellular chromatin and phosphatidylserine on APL cells provide platforms for fibrin deposition and render clots more resistant to fibrinolysis. Additionally, coincubation assays revealed that promyelocytic extracellular chromatin is cytotoxic to ECs, converting them to a procoagulant phenotype. This cytotoxity was blocked by DNase I by 20% or activated protein C by 31%. Our current results thus delineate the pathogenic role of promyelocytic extracellular chromatin in APL coagulopathy. Furthermore, the remaining coagulation disturbance in high-risk APL patients after ATRA administration may be treatable by intrinsic pathway inhibition via accelerating extracellular chromatin degradation.
Introduction
Acute promyelocytic leukemia (APL) is characterized by life-threatening coagulopathy consisting of thrombotic and bleeding complications.1-3 Thanks to the application of all-trans-retinoic acid (ATRA) during the last 2 decades, APL has been considered a highly curable disease with >90% remission rate.4,5 Disappointingly, the early death rate still remains at 17% to 29% in population-based registries (with coagulopathy accounting for 40% to 65% of these cases) despite ATRA administration.1-3 Thus, further understanding of the pathogenesis of APL coagulopathy is urgently needed.
ATRA has been shown to rapidly reverse signs of coagulation, reduce blood product consumption, and reduce bleeding severity through downregulation of well-known procoagulants such as tissue factor (TF), cancer procoagulant, and annexin II on both APL cells and human APL cell line NB4.6-8 However, severe bleeding or thrombotic complications still occur even when rapid administration of ATRA is given at the first suspicion of APL.3,9 Furthermore, previous trials have reported that clotting activation markers and fibrinogen often did not return to normal even when the clinical bleeding diathesis is resolved.8,10 It is attractive to speculate whether other unknown procoagulants exist that cannot be attenuated by ATRA.
Previous studies suggested that cell-free DNA (cf-DNA), from apoptotic cells or in the form of neutrophil extracellular traps (NETs), may activate coagulation via the contact pathway11-15 and thereby enhance the pathology of various coagulation-associated diseases such as sepsis,16 acute myocardial infarction,17 and acute venous thromboembolism.18 Recently, we have shown that APL blasts undergo ETosis, a novel cell death pathway distinct from apoptosis or necrosis, which releases intact chromatin into the extracellular space in response to stimulation.19 However, relatively little is known about the role of promyelocytic extracellular chromatin in APL coagulopathy or its response to ATRA treatment.
Here we continued our previous study by evaluating how extracellular chromatin influences the fibrinolytic and procoagulant activity (PCA) of APL cells and observing the distribution of fibrin on APL cells undergoing ETosis or apoptosis after ATRA treatment. Moreover, because endothelium damage can initiate differentiation syndrome (DS) and exacerbate coagulopathy, the interaction between promyelocytic extracellular chromatin and endothelial cells (ECs) was also explored. Our study may help identify novel targets for coagulopathy intervention and prevention of early death for APL patients following ATRA administration.
Materials and methods
Patients
Forty newly diagnosed APL patients admitted to the First and Second Affiliated Hospital of Harbin Medical University from October 2014 to November 2016 were studied after informed consent. The diagnosis was based on clinical data, morphology, cytochemistry, immunology, cytogenetics, and molecular pathology testing or alternatively confirmation of the presence of the t (15; 17) (PML-RARA) fusion gene.20 This study was approved by the Ethics Committee of Harbin Medical University and conducted according to the Declaration of Helsinki. The main characteristics of the patients on the day of bone marrow aspiration are shown in Table 1.
Reagents
Human APL NB4 cell line was a gift from James O’kelly (Los Angeles, CA). Human umbilical vein endothelial cells (HUVECs), ECs medium, and poly-l-lysine were from ScienCell (San Diego, CA). RPMI 1640 medium and fetal bovine serum were obtained from Gibco (Grand Island, NY). Ficoll-Hypaque, bovine serum albumin, tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), IL-6, ATRA, daunorubicin, EDTA, Triton X-100, and DNase I were all obtained from Sigma-Aldrich (St. Louis, MO). Human recombinant activated protein C (APC) was obtained from Eli Lilly (IN). Propidium iodide (PI) was from Shanghai Dobio Co. Ltd. (Shanghai, China). Spectrozyme-PL (H-d-norleucyl-hexahydrotyrosyl-lysine-p-nitroanilide), tissue type plasminogen activator (t-PA), polyclonal anti-human TF (4502), and fluorescein isothiocyanate (FITC)–conjugated anti-human TF (4508CJ) were all obtained from American Diagnostica (Stamford, CT). Human α-thrombin (IIa); human factor X (FX), FXa, FIXa, FVIIa, and FVIII; and thrombin were obtained from Enzyme Research Laboratories (South Bend, IN). Human factor V (FV), FVa, fluorescein Glu-Gly-Arg-chloromethylketone (EGRCK), and lactadherin were all obtained from Haematologic Technologies (Burlington, VT). Chromogenic substrates S-2765 and S-2238 were obtained from Instrumentation Laboratory Company (MA). Alexa Fluor 488 or 647–conjugated lactadherin, fluorescein-labeled fibrinogen, FVa, and FXa were prepared in our laboratory.
Promyelocytic extracellular traps stimulation, quantification, and isolation
Isolated APL cells and NB4 cells were resuspended in RPMI 1640, and 1 × 106 cells were seeded per well in 6-well plates. Cells were primed with cytokine mixture of TNF-α (10 ng/mL), IL-1β (10 ng/mL), and IL-6 (10 ng/mL) for 1 hour at 37°C. The medium was removed, and wells were washed with RPMI. Cells were then treated with 1 μM ATRA or phosphate buffered saline (PBS) for the indicated time points (0, 1, 3, 5 days) at 37°C. Extracellular chromatin isolation was performed as previously described.17,21 To characterize cell death, APL or NB4 cells were incubated with PI and FITC-labeled lactadherin.22 Cells were washed and analyzed immediately on a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss Jena GmbH, Jena, Germany). Samples were excited with the 488 nm emission line of a krypton-argon laser. Cells undergoing ETosis were identified by rounded morphology, PI staining, and the presence of nuclear content diffused throughout the cell.19 Cells were counted from 6 random fields in triplicate wells for each condition and expressed as percentage of total number of cells in the field.23
Determination of cf-DNA, MPO-DNA complex, and thrombin-antithrombin complex levels in the supernatant
cf-DNA was quantified in the supernatant and plasma of newly diagnosed APL patients using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen) according to the manufacturer’s instructions. Extracellular chromatin from ATRA-treated APL cells on day 3 was ∼800 ng/mL. The 20- and 50-fold concentrated extracellular chromatin from ATRA-treated APL/NB4 cells used in the indicated experiments was ∼16 μg/mL and 40 μg/mL, respectively. Myeloperoxidase-DNA (MPO-DNA) complex was detected in the supernatant and plasma using a capture enzyme-linked immunosorbent assay (ELISA) as previously described.17,19,24,25 Thrombin-antithrombin (TAT) complex was detected by ELISA in control plasma incubated with isolated extracellular chromatin in vitro as previously described.17
ECs stimulation assays
ECs were incubated in RPMI in the presence or absence of concentrated extracellular chromatin (20-fold concentrated) derived from NB4 cells treated by PBS or ATRA (day 3) at room temperature for 24 hours. For inhibition assays, isolated extracellular chromatin was pretreated with DNase I (100 U/mL)26 for 20 minutes or APC (100 nM)21 for 1 hour at 37°C prior to its introduction into culture supernatant of HUVECs. At designated time points, ECs were centrifuged and washed twice with PBS for the following experiments. Phosphatidylserine (PS) exposure was detected by flow cytometer. Prothrombinase, intrinsic FXa, and extrinsic FXa assays were performed as previously described.26
PCA and fibrin formation assays
PCA of APL cells after ATRA treatment was evaluated by 1-stage recalcification time assay in a KC4A-coagulometer (Amelung, Labcon, Heppenheim, Germany).27 Fibrin formation on APL cells was quantified by turbidity as described.28 Briefly, cell-containing suspensions (25 μL 1 × 106 cells were washed twice and resuspended in 75 μL of Tyrode’s buffer) were incubated with prewarmed microparticle-depleted plasma (MDP) (20%) from healthy controls in the presence of 3 mM calcium. For the inhibition assays, isolated extracellular chromatin was pretreated with lactadherin (128 nM), anti-TF (40 μg/mL) for 10 minutes or DNase I (100 U/mL) for 20 minutes at 37°C before incubation with plasma. After incubation for 3 minutes, 100 μL of preheated 25 mM CaCl2 was added, and the time to fibrin strand formation was recorded.
After incubation with isolated extracellular chromatin, HUVECs were rinsed with Tyrode’s buffer and then were overlaid with prewarmed MDP (15%) from healthy controls in the presence of 3 mM calcium. Fibrin production was measured by turbidity at 405 nm in a SpectraMax 340PC plate reader. All clotting assays were performed in triplicate.
Confocal microscopy
PS exposure on cultured ECs was determined by incubation of HUVECs with Alexa Fluor 488–labeled lactadherin and Alexa Fluor 647–labeled CD31. To observe FXa and FVa binding, stimulated ECs and NB4 cells were costained with FVa-fluorescein-maleimide and FXa-EGRck-biotin (complexed to Alexa 647–streptavidin). Fibrin networks on stimulated ECs and NB4 cells were stained by Alexa Fluor 647–conjugated fibrinogen and Alexa Fluor 488–labeled lactadherin. All of the previous samples were excited with 488 or 568 nm emission lines of a krypton-argon laser, and narrow band pass filters were used for restricting emission wavelength overlap. Images were obtained using an LSM 510 System. Background signal was calculated by using a similarly labeled isotype matched control antibody.
Plasminogen activation assay
Plasminogen activation assay was performed as previously described.29
Plasma clot lysis assay
Plasma clot lysis assay was performed as previously described.30
Clot permeability assays
Clots were prepared from 8 μM fibrinogen supplemented with 20 mM Ca2+ ± extracellular chromatin (50-fold concentrated) and were clotted with 16 nM thrombin in plastic pipette tips. After 70 minutes of incubation at 37°C, PBS was permeated through the clots. Pressure was kept constant by maintaining a fixed head volume (pressure drop was 0.056 N/cm). Permeability coefficient (Ks) was calculated as previously described.31
The methods for “Blood collection,” “Cell culture,” “Plasminogen activation assay,” and “Plasma clot lysis assay” are presented in detail in the supplemental Methods (available on the Blood Web site).
Statistical analysis
Numerical variables were tested for normal distribution with the Kolmogorov-Smirnov test. Normally distributed variables were summarized as mean ± SD, and statistical analysis was made by t test or analysis of variance as appropriate. Nonnormally distributed variables were summarized as medians with interquartile ranges, and the Mann-Whitney U test was conducted. Correlations between 2 continuous variables were performed using Pearson correlation coefficients, and the Spearman rank correlation was used to detect discrete variables. Categorical variables were compared using the χ2 test. P < .05 was considered statistically significant.
Results
ATRA potentiates procoagulant extracellular chromatin release from APL and NB4 cells
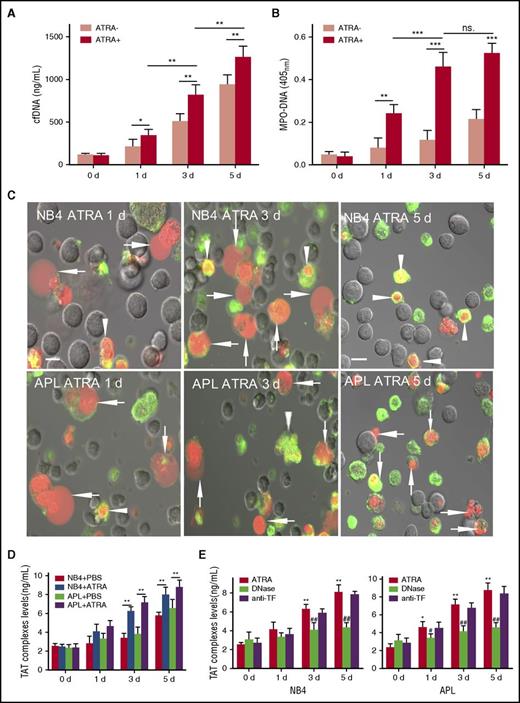
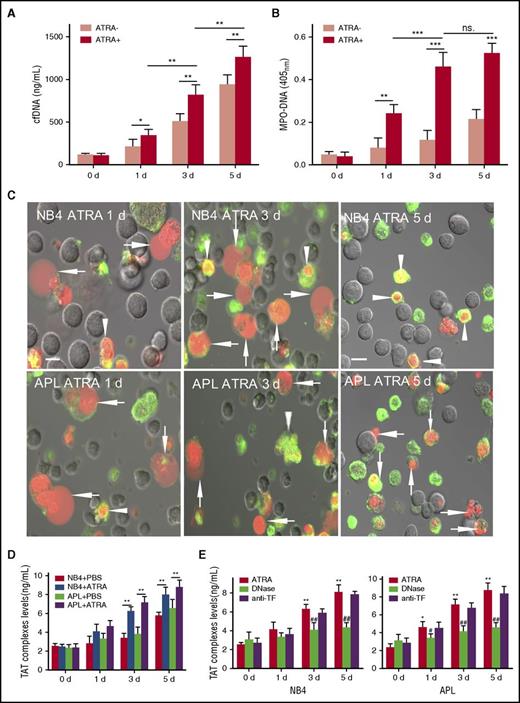
ATRA treatment induced markedly increased cf-DNA release in a time-dependent manner compared with the untreated group (Figure 1A) (NB4 data not shown). MPO-DNA, a marker of ETosis,24,25 was higher in the ATRA-treated cells than in controls. Additionally, MPO-DNA showed no significant increase from day 3 to day 5, indicating that the increase in cf-DNA during this time was mainly from apoptosis (Figure 1B). To determine the predominant cell death pattern that is responsible for the increased extracellular chromatin, APL/NB4 cells were stained with lactadherin and PI and analyzed by confocal microscopy (Figure 1C). Results showed that ETosis was the major cell death pattern seen in the ATRA-treated group up to the third day, indicating that the increase in cf-DNA triggered by ATRA was mainly from ETosis. Apoptosis was predominant in the no-treatment group on day 3, consistent with our previous study.19 Extracellular chromatin showed no significant degradation when incubated with control plasma for up to 45 minutes (supplemental Figure 1). Thrombin generation in the presence of extracellular chromatin isolated from all cells progressively increased over time. Extracellular chromatin isolated from the ATRA treatment group increased thrombin generation approximately twofold on day 3 compared with that from the untreated group and 1.5-fold on day 5 (Figure 1D). Degrading cf-DNA using DNase I decreased thrombin generation by 42% on day 3 and 51% on day 5 for APL cells. For NB4 cells, DNase inhibited thrombin generation by 36% on day 3 and 47% on day 5, indicating a relatively strong procoagulant effect of promyelocytic cf-DNA. Neutralizing anti-TF antibody had no effect (Figure 1E). cf-DNA and MPO-DNA complexes were measured in the peripheral blood of newly diagnosed APL patients. Patients had markedly increased levels of cf-DNA and MPO-DNA complexes compared with controls. Further, baseline WBC counts were positively correlated to both plasma cf-DNA and MPO-DNA complexes (supplemental Figure 2A).
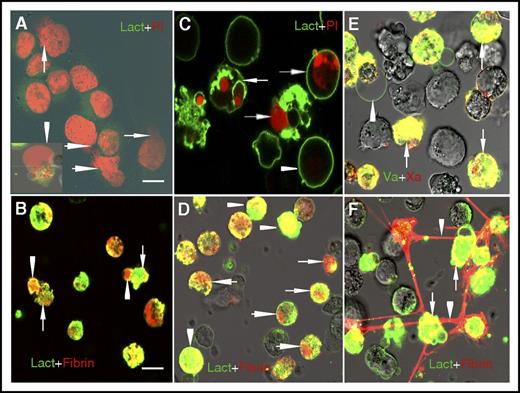
ATRA induces APL/NB4 cells to release procoagulant extracellular chromatin. Fresh APL cells were primed with a mix of cytokines (10 ng/mL TNF-α, 10 ng/mL IL-1β, and 10 ng/mL IL-6) for 1 hour and then were incubated with 1 μM ATRA. Levels of cf-DNA (A) and MPO-DNA complexes (B) in the supernatant were measured at the indicated time points. (C) Representative confocal microscopy images of APL/NB4 cells stained by lactadherin (green) and PI (red). Cells with expanded nuclei that lost shape and filled most of the cytoplasm were counted as ETs releasing cells (arrow). Those with condensed and fragmented nuclei were counted as apoptotic cells (arrowhead). Bars represent 10 μm. One out of 6 independent experiments is shown. (D) Promyelocytic extracellular chromatin was isolated and incubated with 20% plasma from healthy controls. TAT complexes were measured by ELISA. (E) For inhibition assays, isolated extracellular chromatin was pretreated with DNase I or anti-TF antibody before incubation with plasma. Data are from 6 independent experiments and presented as means ± SD. *P < .05, **P < .01, ***P < .001 vs day 0; #P < .05, ##P < .001 vs no inhibitor group in E.
ATRA induces APL/NB4 cells to release procoagulant extracellular chromatin. Fresh APL cells were primed with a mix of cytokines (10 ng/mL TNF-α, 10 ng/mL IL-1β, and 10 ng/mL IL-6) for 1 hour and then were incubated with 1 μM ATRA. Levels of cf-DNA (A) and MPO-DNA complexes (B) in the supernatant were measured at the indicated time points. (C) Representative confocal microscopy images of APL/NB4 cells stained by lactadherin (green) and PI (red). Cells with expanded nuclei that lost shape and filled most of the cytoplasm were counted as ETs releasing cells (arrow). Those with condensed and fragmented nuclei were counted as apoptotic cells (arrowhead). Bars represent 10 μm. One out of 6 independent experiments is shown. (D) Promyelocytic extracellular chromatin was isolated and incubated with 20% plasma from healthy controls. TAT complexes were measured by ELISA. (E) For inhibition assays, isolated extracellular chromatin was pretreated with DNase I or anti-TF antibody before incubation with plasma. Data are from 6 independent experiments and presented as means ± SD. *P < .05, **P < .01, ***P < .001 vs day 0; #P < .05, ##P < .001 vs no inhibitor group in E.
Effect of ATRA on integrated PCA of APL cells
Having identified a procoagulant effect of isolated soluble chromatin, we next tested the overall effect of ATRA treatment of various incubation times on PCA and fibrin formation when APL cells were mixed with plasma. ATRA differentiation resulted in a marked decrease in PCA and fibrin formation of cytokine-primed APL cells on days 1 to 3 compared with day 0 followed by an increase on day 5. In contrast, the PCA and fibrin formation of PBS-treated APL cells progressively increased during the 5 days. In comparison with the PBS-treated group, ATRA-treated APL cells exhibited lengthened coagulation time and decreased fibrin formation during the 5 days (Figure 2A-B). For inhibition assays, cells were mixed with either DNase I, anti-TF, or lactadherin prior to coagulation time or fibrin formation assays. Lactadherin caused a significant reduction in PCA and fibrin formation at all time points for both ATRA- and PBS-treated cells. DNase I reduced PCA and fibrin formation for ATRA-treated cells on day 3 and day 5 (Figure 2C-D) while having no effect on PBS-treated cells. Anti-TF had minimal effect on ATRA-treated cells but dramatically decreased PCA and fibrin formation on untreated cells (Figure 2E-F).
Effect of ATRA on integrated PCA of cytokine-primed APL cells. Cytokine-primed APL cells were treated with or without ATRA for the indicated time and then incubated with MDP from healthy controls. (A) Coagulation time was measured using a recalcification-time assay. (B) Fibrin production was measured by turbidity at 405 nm (optical density 405 [OD405]). (C-D) For inhibition assays, ATRA-differentiated APL cells were treated with DNase I, anti-TF antibody, or lactadherin before incubation with plasma. Coagulation time and fibrin formation were then evaluated. (E-F) Inhibition assays were also performed using APL cells treated with PBS as a control. Data are representative of 4 independent experiments and are displayed as mean ± SD. *P < .05, **P < .01 vs day 0; #P < .05, ##P < .01 vs ATRA-treated group in panels A-B; *P < .01, **P < .001 vs no inhibitor treated group in panels C-F.
Effect of ATRA on integrated PCA of cytokine-primed APL cells. Cytokine-primed APL cells were treated with or without ATRA for the indicated time and then incubated with MDP from healthy controls. (A) Coagulation time was measured using a recalcification-time assay. (B) Fibrin production was measured by turbidity at 405 nm (optical density 405 [OD405]). (C-D) For inhibition assays, ATRA-differentiated APL cells were treated with DNase I, anti-TF antibody, or lactadherin before incubation with plasma. Coagulation time and fibrin formation were then evaluated. (E-F) Inhibition assays were also performed using APL cells treated with PBS as a control. Data are representative of 4 independent experiments and are displayed as mean ± SD. *P < .05, **P < .01 vs day 0; #P < .05, ##P < .01 vs ATRA-treated group in panels A-B; *P < .01, **P < .001 vs no inhibitor treated group in panels C-F.
Distribution of fibrin on NB4 cells undergoing apoptosis or ETosis after ATRA treatment
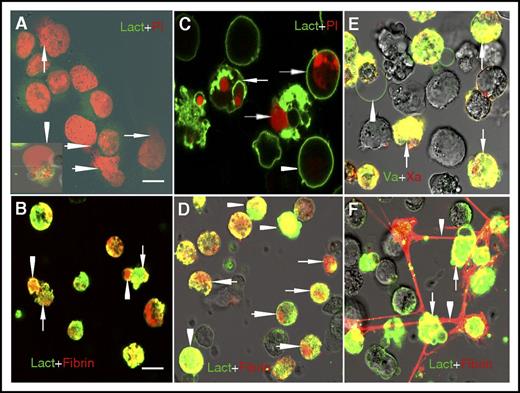
Because extracellular chromatin causes elevated fibrin generation, we then explored how fibrin is distributed during ETosis or apoptosis using confocal microscopy. For cells undergoing ETosis, the nuclei expanded and filled with cytoplasm. Then the cells expanded into a single bubble that was larger than the quiescent cells, with a diffuse rim weakly stained by lactadherin. When the thinner membrane of the bubble was damaged, the chromatin spilled out into the extracellular space (Figure 3A). Fibrin deposited on the same position of expanded chromatin inside the single bubble. Furthermore, fibrin (red) also deposited around the bubble, colocalized with exposed PS on the thin intact membrane and the remaining broken membrane fragments (Figure 3B). On day 5, most cells underwent apoptosis, with clear staining of the nuclear and externalized PS on the cell membrane (Figure 3C). We observed fibrin deposition at the same position as the compromised chromatin inside the apoptotic cells. Moreover, fibrin also colocalized with externalized PS on the membrane which was strongly stained by lactadherin (Figure 3D). Staining of FXa (red) and FVa (green) showed patchy distributions that overlapped (yellow) on apoptotic NB4 cell membranes (Figure 3E), indicating Xa/Va (prothrombinase) complex assembly. ET-releasing cells also showed substantial FVa deposition on the thin membrane around the bubble. Both FXa and FVa share a preference for convex surfaces, similar to lactadherin. For some adjacent late apoptotic NB4 cells, a large amount of fibrin (red) strands deposited on the membranes with exposed PS (green), forming a net structure among NB4 cells (Figure 3F).
Different topographical distribution of fibrin on ATRA-treated NB4 cells undergoing apoptosis and ETosis. (A) On the third day of ATRA treatment, NB4 cells undergoing ETosis were stained with lactadherin (Lact; green) and PI (red). Decondensed extracellular chromatin could be seen enclosed by a thin layer of membrane (arrowhead) or spilled out of the ruptured cell (arrow). (B) Fibrin deposited on the diffuse decondensed chromatin within the bubble and also along the thin membrane around the bubble (arrowhead) and on the remaining broken cell membrane (arrow), similar to the binding sites for lactadherin. (C) On day 5, ATRA treatment led to diffuse rim staining by lactadherin on most cells undergoing early apoptosis (arrowhead). Late apoptotic cells stained with lactadherin and had condensed nuclei stained with PI (arrow). (D) Fibrin deposited at the same position as condensed chromatin inside the apoptotic cells (arrow) and also colocalized with externalized PS on the membrane that could be identified by strong staining with lactadherin (arrowhead). (E) FXa (red) and FVa (green) had patchy coherent distributions that overlapped on apoptotic NB4 cell membrane (arrow). FVa staining was observed on the thin membrane around ETs releasing cells (arrowhead). (F) A large amount of fibrin (red) strands deposited on the adjacent late apoptotic APL cells (arrow) and formed a net of fibrin (arrowhead). Representative images of 6 independent experiments are shown. Bars represent 10 μm in panels A, C, E; 20 μm in panels B, D, F.
Different topographical distribution of fibrin on ATRA-treated NB4 cells undergoing apoptosis and ETosis. (A) On the third day of ATRA treatment, NB4 cells undergoing ETosis were stained with lactadherin (Lact; green) and PI (red). Decondensed extracellular chromatin could be seen enclosed by a thin layer of membrane (arrowhead) or spilled out of the ruptured cell (arrow). (B) Fibrin deposited on the diffuse decondensed chromatin within the bubble and also along the thin membrane around the bubble (arrowhead) and on the remaining broken cell membrane (arrow), similar to the binding sites for lactadherin. (C) On day 5, ATRA treatment led to diffuse rim staining by lactadherin on most cells undergoing early apoptosis (arrowhead). Late apoptotic cells stained with lactadherin and had condensed nuclei stained with PI (arrow). (D) Fibrin deposited at the same position as condensed chromatin inside the apoptotic cells (arrow) and also colocalized with externalized PS on the membrane that could be identified by strong staining with lactadherin (arrowhead). (E) FXa (red) and FVa (green) had patchy coherent distributions that overlapped on apoptotic NB4 cell membrane (arrow). FVa staining was observed on the thin membrane around ETs releasing cells (arrowhead). (F) A large amount of fibrin (red) strands deposited on the adjacent late apoptotic APL cells (arrow) and formed a net of fibrin (arrowhead). Representative images of 6 independent experiments are shown. Bars represent 10 μm in panels A, C, E; 20 μm in panels B, D, F.
Promyelocytic extracellular traps impair fibrinolysis
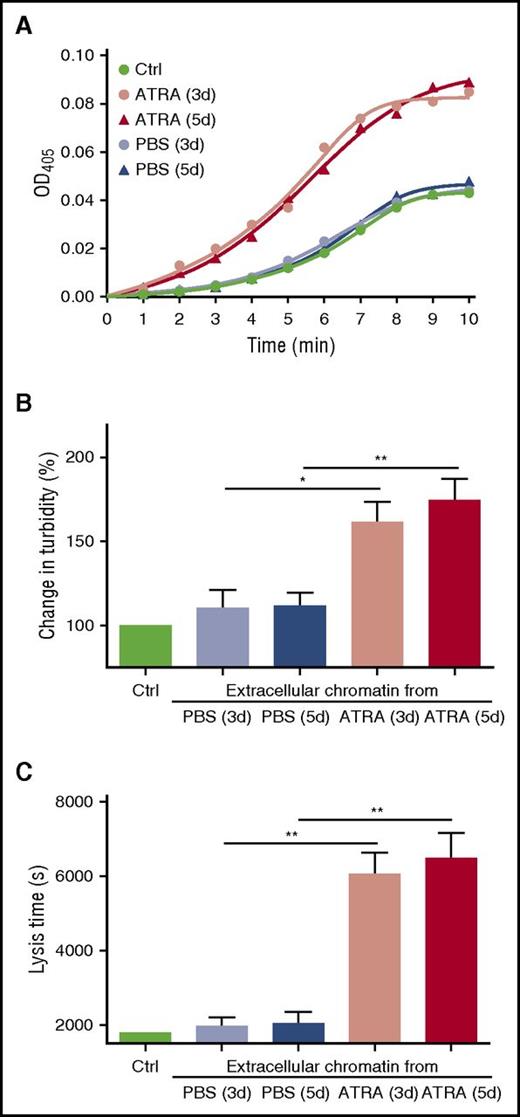
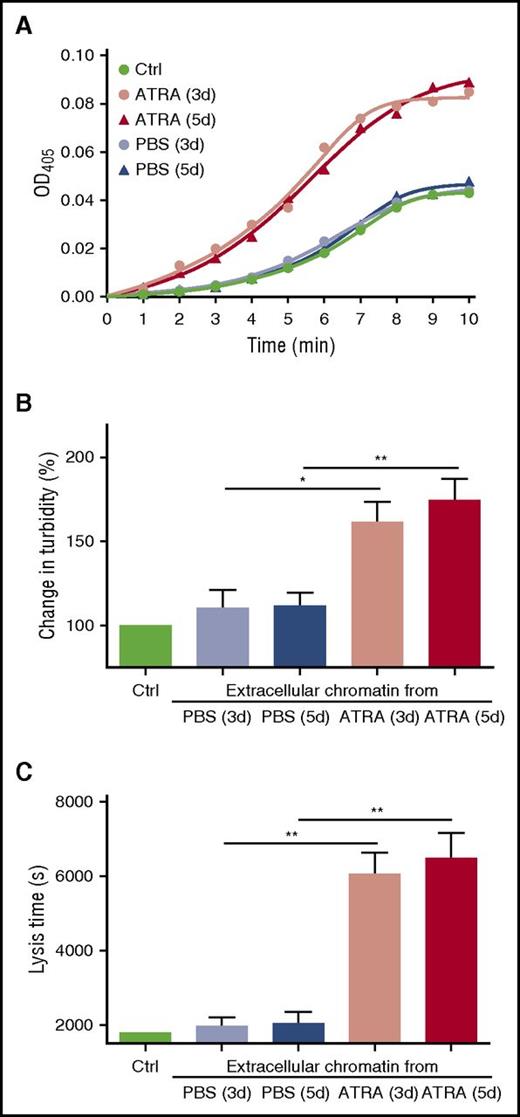
Because fibrin bound to chromatin (extracellular and intracellular) in the microscopy experiments, we investigated whether promyelocytic chromatin influences the structure of fibrin and the process of fibrinolysis such as plasminogen activation and clot digestion. Plasma clots formed in the presence of concentrated chromatin from ATRA-treated or PBS-treated APL cells were mixed with t-PA and the generation of plasmin was measured. A titration curve of plasmin generation using different concentrations of extracellular chromatin was performed to find an appropriate experimental concentration (supplemental Figure 3). Concentrated chromatin from the ATRA-treated cells markedly enhanced plasmin generation, whereas chromatin from the PBS-treated cells had little effect compared with control clots formed without chromatin. Although the amount of chromatin peaked on day 5 (Figure 1A), plasmin generation from the ATRA-treated cells on day 5 was not significantly different than seen on day 3 (Figure 4A), suggesting different effects from chromatin resulting from ETosis vs apoptosis. Clot lysis assays were performed on control plasma supplemented with isolated chromatin. Addition of chromatin from the ATRA-treated group to control plasma increased maximum absorbance by ∼1.64-fold (Figure 4B) and caused a 3.03-fold prolongation of the clot lysis time compared with PBS-treated groups (Figure 4C). In the clot permeability assays, chromatin from the ATRA-treated groups increased the permeability of clots by ∼3.3-fold, whereas chromatin from PBS-treated groups had no significant effect (Table 2). No significant difference in maximum absorbance, clot lysis, or clot permeability assays were seen between chromatin isolated from APL cells on day 3 and day 5 of ATRA treatment. For newly diagnosed APL patients, plasma cf-DNA was negatively correlated with fibrinogen. No correlation was found between cf-DNA or MPO-DNA complexes with D-dimer (supplemental Figure 2B).
Promyelocytic extracellular chromatin enhances plasmin generation and fibrin structure. (A) Plasma containing plasminogen and isolated concentrated extracellular chromatin were prepared. t-PA and the plasmin substrate Spectrozyme-PL were then added and the absorbance of the liberated p-nitroaniline was continuously measured at 405 nm (OD405). The figure shows mean values of triplicate measurements from 6 independent experiments. Clot lysis assays were performed in normal plasma supplemented with isolated concentrated extracellular chromatin in the presence of 1 nM t-PA. Changes in clot structure were measured by observing clot turbidity at 405 nm (B); lysis time was recorded (C). Each point represents mean ± SD for triplicate samples of independent experiments. *P < .05, **P < .01.
Promyelocytic extracellular chromatin enhances plasmin generation and fibrin structure. (A) Plasma containing plasminogen and isolated concentrated extracellular chromatin were prepared. t-PA and the plasmin substrate Spectrozyme-PL were then added and the absorbance of the liberated p-nitroaniline was continuously measured at 405 nm (OD405). The figure shows mean values of triplicate measurements from 6 independent experiments. Clot lysis assays were performed in normal plasma supplemented with isolated concentrated extracellular chromatin in the presence of 1 nM t-PA. Changes in clot structure were measured by observing clot turbidity at 405 nm (B); lysis time was recorded (C). Each point represents mean ± SD for triplicate samples of independent experiments. *P < .05, **P < .01.
Cytotoxic effect of promyelocytic extracellular chromatin on HUVECs
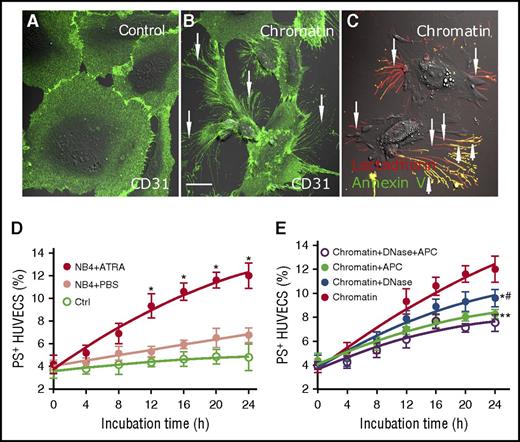
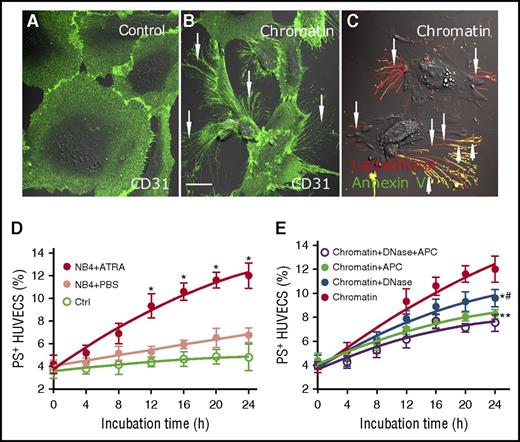
In response to lung injury, neutrophil extracellular traps and histones induce activation and apoptosis of ECs.21,32 We therefore wondered whether isolated extracellular chromatin from NB4 cells has a cytotoxic effect on HUVECs. Titration curve of % PS+ HUVECs triggered by different concentrations of isolated extracellular chromatin for 24 hours was performed to find an appropriate experimental concentration (supplemental Figure 4). ECs treated with ATRA-derived extracellular chromatin lost their normal morphology and retracted from the cell-cell junctions obviously (Figure 5B), whereas changes of ECs treated with PBS extracellular chromatin were minimal (Figure 5A). Filopodia and localized regions on the EC margins costained with Alexa Fluor 647–lactadherin and Alexa Fluor 488–annexin V, indicating PS exposure (Figure 5C). Results showed that ATRA-derived chromatin triggered an increase in PS exposure on ECs in a time-dependent manner compared with PBS-derived chromatin (Figure 5D). Inhibition studies were performed using DNase I to degrade the DNA scaffold and APC to dissect histones. PS exposure on ECs was inhibited by 20%, 31%, and 37.2% when DNase I, APC, or both, respectively, were included in the assay, indicating that the DNA scaffold and histones are both necessary for the cytotoxic effect of extracellular chromatin. Additionally, the combined inhibitory effect of DNase I and APC was significantly stronger than DNase I alone (P < .05) (Figure 5E).
Promyelocytic extracellular chromatin triggers PS exposure on HUVECs. On the third day of PBS or ATRA treatment, extracellular chromatin from NB4 cells was isolated. HUVECs were incubated with or without isolated extracellular chromatin (50-fold concentrated) for 24 hours. Cells were stained with CD31-Alexa Fluor 488 (A-B) and with Alexa 647–lactadherin and FITC–annexin V (C). (A) Representative confocal microscopy image showed morphology of HUVECs incubated with culture medium without extracellular chromatin. (B-C) Stimulation with ATRA-derived chromatin led to the retraction of cell margins and extension of filopods (arrows) and PS exposure on the filopods (arrows), which costained with lactadherin (red) and annexin V (green). The inset bar represents 5 μm in panels A-C. One of 6 independent experiments is shown. (D) Kinetics of PS reversal on ECs with different treatment. (E) For inhibition assays HUVECs were incubated with isolated ATRA-derived chromatin in the presence or absence of DNase I or APC. One of 6 independent experiments is shown. Each point represents mean ± SD. *P < .001 vs PBS-treated group in panel D; *P < .01, **P < .001 vs no inhibitor group and #P < .05 vs DNase-treated group in panel E.
Promyelocytic extracellular chromatin triggers PS exposure on HUVECs. On the third day of PBS or ATRA treatment, extracellular chromatin from NB4 cells was isolated. HUVECs were incubated with or without isolated extracellular chromatin (50-fold concentrated) for 24 hours. Cells were stained with CD31-Alexa Fluor 488 (A-B) and with Alexa 647–lactadherin and FITC–annexin V (C). (A) Representative confocal microscopy image showed morphology of HUVECs incubated with culture medium without extracellular chromatin. (B-C) Stimulation with ATRA-derived chromatin led to the retraction of cell margins and extension of filopods (arrows) and PS exposure on the filopods (arrows), which costained with lactadherin (red) and annexin V (green). The inset bar represents 5 μm in panels A-C. One of 6 independent experiments is shown. (D) Kinetics of PS reversal on ECs with different treatment. (E) For inhibition assays HUVECs were incubated with isolated ATRA-derived chromatin in the presence or absence of DNase I or APC. One of 6 independent experiments is shown. Each point represents mean ± SD. *P < .001 vs PBS-treated group in panel D; *P < .01, **P < .001 vs no inhibitor group and #P < .05 vs DNase-treated group in panel E.
Promyelocytic extracellular traps convert HUVECs to a procoagulant phenotype
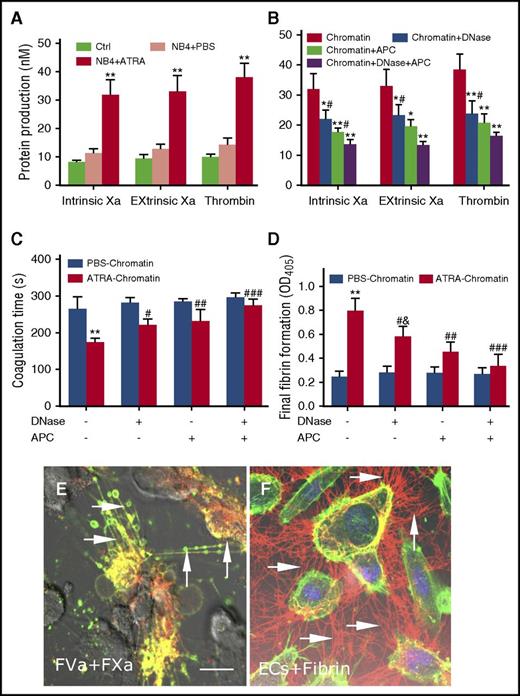
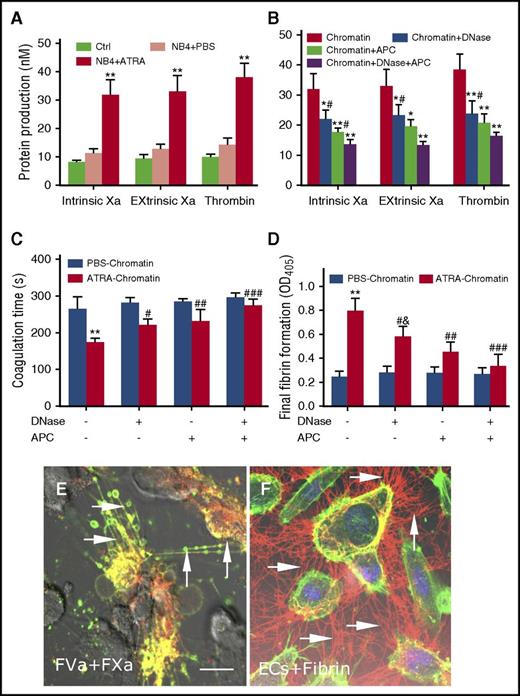
Because ATRA-derived extracellular chromatin induced increased PS exposure on ECs, we then speculated whether the increased PS exposure can support elevated tenase and prothrombinase activity. Data showed that compared with those treated by PBS-derived chromatin, ECs incubated with ATRA-derived chromatin increased production of intrinsic and extrinsic FXa complexes and thrombin generation (Figure 6A). For the inhibition assays: thrombin and intrinsic and extrinsic FXa production were reduced by 38%, 31.1%, and 29.5%, respectively, in the presence of DNase I; by 46%, 44.7%, and 40.7% with APC; and by 57.5%, 57.6%, and 59.4% with both DNase I and APC (Figure 6B). Next, the integrated PCA of ECs was evaluated using coagulation time and fibrin generation assays. As expected, treatment of ECs with ATRA-derived chromatin triggered rapid and massive fibrin formation, which accelerated clotting (Figure 6C). Additionally, combination of DNase I and APC produced a significantly stronger inhibitory effect than DNase I alone, indicating that although the DNA scaffold is necessary, the cytotoxicity of extracellular chromatin was mainly because of histones (Figure 6D). Using confocal microscopy, a significant colocalized fraction of bound FVa and FXa was observed, indicating that ECs treated with ATRA extracellular chromatin were able to offer a biological surface for binding coagulation factors, most likely through externalized PS (Figure 6E). Furthermore, large fibrin strands were radially distributed along the filopodia of ECs that were pretreated with ATRA extracellular chromatin (Figure 6F).
Promyelocytic extracellular chromatin converts HUVECs into a procoagulant phenotype. (A) Thrombin and intrinsic Xa and extrinsic Xa production were measured on HUVECs treated with NB4 extracellular chromatin from the different treatment groups. (B) Inhibition assays of protein production were performed using DNase I or APC to degrade extracellular chromatin before incubation with ECs. Coagulation time (C) and fibrin formation (D) of ECs stimulated with ATRA- or PBS-derived NB4 chromatin for 24 hours in the presence of DNase I or APC were measured. Data shown from 3 independent experiments and presented as mean ± SD. (E) FXa (red) and FVa (green) costaining (yellow) was observed on filopods near the retracted edges of ECs and on newly formed thin filaments (arrow), similar to the binding sites for lactadherin. (F) ECs pretreated with ATRA-derived chromatin and incubated with healthy plasma showed considerable fibrin strand formation arranged radially along with filopodia (arrow), which formed a fibrin network. The inset bar represents 5 μm in panels E-F. One of 6 independent experiments is shown. *P < .01, **P < .001 vs no inhibitor group and #P < .05 vs DNase I (+) APC (+) in panel B; **P < .001 vs PBS-treated NB4 cells in panels A, C, D; #P < .05, ##P < .01, ###P < .001 vs DNase I (-) APC (-) in panels C-D; &P < .05 vs DNase I (+) APC (+) in panel D.
Promyelocytic extracellular chromatin converts HUVECs into a procoagulant phenotype. (A) Thrombin and intrinsic Xa and extrinsic Xa production were measured on HUVECs treated with NB4 extracellular chromatin from the different treatment groups. (B) Inhibition assays of protein production were performed using DNase I or APC to degrade extracellular chromatin before incubation with ECs. Coagulation time (C) and fibrin formation (D) of ECs stimulated with ATRA- or PBS-derived NB4 chromatin for 24 hours in the presence of DNase I or APC were measured. Data shown from 3 independent experiments and presented as mean ± SD. (E) FXa (red) and FVa (green) costaining (yellow) was observed on filopods near the retracted edges of ECs and on newly formed thin filaments (arrow), similar to the binding sites for lactadherin. (F) ECs pretreated with ATRA-derived chromatin and incubated with healthy plasma showed considerable fibrin strand formation arranged radially along with filopodia (arrow), which formed a fibrin network. The inset bar represents 5 μm in panels E-F. One of 6 independent experiments is shown. *P < .01, **P < .001 vs no inhibitor group and #P < .05 vs DNase I (+) APC (+) in panel B; **P < .001 vs PBS-treated NB4 cells in panels A, C, D; #P < .05, ##P < .01, ###P < .001 vs DNase I (-) APC (-) in panels C-D; &P < .05 vs DNase I (+) APC (+) in panel D.
Discussion
In this study, we made 4 significant observations. First, thrombin generation paralleled the release of promyelocytic extracellular chromatin induced by ATRA. Moreover, on the third day of ATRA treatment, DNase I reduced PCA and fibrin generation, whereas anti-TF antibody produced no effect. For untreated APL/NB4 cells, anti-TF antibody significantly inhibited PCA and fibrin generation, whereas the effect of DNase I was minimal. Second, confocal microscopy showed that fibrin preferentially deposits on promyelocytic chromatin from ETosis or apoptosis and exposed PS. Notably, extracellular chromatin from ETosis, but not apoptosis, promotes plasmin generation while at the same time impairing clot lysis, thus highlighting the involvement of extracellular chromatin in fibrinolysis. Fourth, isolated promyelocytic extracellular chromatin exerted a strong cytotoxic effect on ECs, converting them to an increased procoagulant phenotype.
NETosis can be triggered by many factors including bacterial infection, inflammatory cytokines, and immune disorder. Particular emphasis has been placed on the harmful effects of increased extracellular chromatin in various disease states. For APL cells, ETosis appears to be the major source of the increased extracellular chromatin in the early period of ATRA treatment, although it is difficult to determine whether ETosis is promoted directly from the drug treatment or indirectly through induced differentiation. However, this effect seems predominantly dependent on increased cytokines from differentiating myeloid cells during ATRA administration, as evidenced by our previous study.19 Patients with infection, disseminated intravascular coagulation (DIC), a high number of leukocytes,33 and differentiation syndrome all present with high systematic inflammatory responses, where enhanced ETosis occurred. Increased cf-DNA and decreased DNase I activity are associated with many coagulopathy-associated diseases such as thrombotic microangiopathies34 and systemic lupus erythematosus.35 Although ATRA successfully suppresses the extrinsic pathway by downregulating the expression of TF and annexin II, it also triggers the release of promyelocytic extracellular chromatin, a potential dangerous factor. Extracellular chromatin may further promote intrinsic coagulation processes, cause excess consumption of coagulation factors such as plasminogen and fibrinogen, enhance fibrinolytic resistance, and damage ECs, contributing to increased incidence of induction failure in high-risk APL patients.
Recent evidence suggests that fibrin, chromatin, and von Willebrand factor form a colocalized network within the thrombus in many thromboembolism diseases such as venous thromboembolism and acute myocardial infarction.17,36 Here, we provided visible evidence that APL cells undergoing ETosis or apoptosis supported fibrin deposition around chromatin (extracellular or intracellular) and accessible PS. These results are supported by a recent study showing high-affinity binding of cf-DNA to fibrinogen and fibrin in a purified system in vitro.30 Of particular interest, we observed that FVa and FXa bind to the PS from APL cells undergoing ETosis or apoptosis, indicating prothrombinase complex assembly. We have previously reported that PS exposure on apoptotic APL cells supports increased generation of intrinsic FXase complex and prothrombinase complex.20 This study goes beyond the prior reports, correlating the release of promyelocytic chromatin and PS exposure from ETosis and apoptosis with increases in prothrombinase complex assembly and fibrin deposition. The combination of increased chromatin with accessible PS could cause excess consumption of coagulation factors and fibrinogen, leading to increased risk of hemorrhage and DIC.
Another interesting finding was that extracellular chromatin produces 2 competing effects in the fibrinolytic system: it directly facilitates t-PA–mediated plasmin generation while also impairing clot lysis. Delayed fibrin lysis could in turn cause continued stimulation of the fibrinolytic system, leading to exacerbated fibrinolysis disorder. Importantly, chromatin from day 5 did not exert a stronger effect on fibrinolysis than that from day 3. It seems that chromatin from apoptotic cells (days 3-5) does not have the same effects on fibrinolysis as chromatin from ETosis (before day 3). This may be because of the special characteristics of the high-molecular-weight fragments of extracellular chromatin from APL cells undergoing ETosis. The size of cf-DNA is known to be dependent on the cellular process by which it was liberated; apoptotic cells release a ladder pattern of DNA at ≈150-bp interval,37 whereas necrotic cells and NET-releasing cells release high-molecular-weight fragments >10 000 bp.38,39 Our results thus reveal the distinct functions of chromatin originating from different death patterns in fibrinolysis. Additionally, extracellular chromatin caused increased cell permeability. Previous research has shown that high levels of cf-DNA decreased the density of clots, whereas low and intermediate levels of cf-DNA increased clot density.30 Furthermore, different concentrations of DNA, histones, or complexes have different effects on fibrin structure.29 Further studies are needed to identify the exact mechanism behind these differences.
DS is a relatively common and serious complication that can occasionally be life threatening in patients with APL undergoing induction therapy with ATRA and/or arsenic trioxide. Endothelium damage is the major pathophysiologic process of DS, leading to local hypoxia, ischemia, and tissue edema, leading to multiple organ failure and DIC.40 A previous study suggested that ATRA enhances the potential of NB4 cells to stimulate the expression of TF and PCA in endothelium, though the mechanism is still not fully known.7 In our study, we demonstrate that extracellular chromatin from ATRA treatment triggered PS exposure on ECs and converted them to a procoagulant phenotype. In addition, our photomicrographs provide visible evidence that ECs treated with ATRA-derived chromatin bound FVa and FXa primarily on the filopods and fibrils of retracted ECs. The selective distribution of fibrin is similar to the preferential binding of prothrombinase to PS. It has been confirmed that NETs derived from neutrophils can cause tissue damage in severe acute pancreatitis41 and sterile inflammatory liver injury.42 However, controversy still remains over whether the DNA scaffold influences the cytotoxic effect of NETs. Saffarzadeh et al showed that NET-mediated cytotoxicity occurs primarily through histones21 and is not influenced by DNA digestion. This controversy may be because of (1) different processes of ETosis occurring in APL/NB4 cells vs polymorphonuclear leukocytes19 or (2) interactions between histones and other enzymes (elastase, MPO)43 because enzymes inside the granules were different between promyelocytic cells and polymorphonuclear leukocytes.44 Moreover, many previous studies have shown that DNase I breakdown of NETs partially attenuates tissue injury, consistent with our results that indicate a cytotoxic role for both histones and the DNA scaffold.32,41,42,45 Our results show that DNase I and APC protect ECs from the cytotoxic effects of promyelocytic extracellular chromatin and decrease the resulting PCA.
We have previously reported that APL plasma triggered enhanced promyelocytic ETosis compared with control plasma.19 In this study, we found a statistically significant positive correlation between baseline WBC counts and both cf-DNA and MPO-DNA. Moreover, cf-DNA was negatively correlated with fibrinogen. Previous studies reported that total white cell count have emerged as good general predictors of hemorrhagic death.46 Because prompt treatment with ATRA, with or without arsenic trioxide, has been the most important step in preventing bleeding complications, future therapeutic strategies could focus on combined application of DNase I and APC with ATRA to accelerate degradation of promyelocytic extracellular chromatin to further decrease risk of coagulopathy, especially in high-risk APL patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Yanming Xue for the sample collection; Jiangtian Tian, Ji Li, and Hulun Li for excellent technical assistance; and James O’Kelly (Los Angeles, CA) for providing NB4 cells.
This work was supported by grants from the National Science Foundation of China (81270588, 81670128, 81670298, 81470301).
Authorship
Contribution: M.C. designed the research, performed experiments, analyzed results, made the figures, and wrote the manuscript; J.S. obtained funding, designed the study, performed experiments, analyzed results, made the figures, and revised the manuscript; J.Z. provided partial funding support; T.L., Z.H., L.W., X.Y., Y.K., L.Z., and X.D. performed some experiments; and V.A.N., Y.B., J.K., B.Y., S.F., and J.W. analyzed data and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jialan Shi, Department of Hematology, The First Hospital, Harbin Medical University, Harbin 150001, China; e-mail: shi73661@gmail.com; Jin Zhou, Department of Hematology, The First Hospital, Harbin Medical University, Harbin 150001, China; e-mail: jin_zhouxy@163.com; Jinghua Wang, Department of Hematology, The Second Hospital, Harbin Medical University, Harbin 150086, China; e-mail: wang_jing_huaxy@163.com; and Shaohong Fang, Key Laboratory of Myocardial Ischemia, Ministry of Education, The Second Hospital, Harbin Medical University, Harbin 150081, China; e-mail: fangshaohong7802@163.com.

![Figure 2. Effect of ATRA on integrated PCA of cytokine-primed APL cells. Cytokine-primed APL cells were treated with or without ATRA for the indicated time and then incubated with MDP from healthy controls. (A) Coagulation time was measured using a recalcification-time assay. (B) Fibrin production was measured by turbidity at 405 nm (optical density 405 [OD405]). (C-D) For inhibition assays, ATRA-differentiated APL cells were treated with DNase I, anti-TF antibody, or lactadherin before incubation with plasma. Coagulation time and fibrin formation were then evaluated. (E-F) Inhibition assays were also performed using APL cells treated with PBS as a control. Data are representative of 4 independent experiments and are displayed as mean ± SD. *P < .05, **P < .01 vs day 0; #P < .05, ##P < .01 vs ATRA-treated group in panels A-B; *P < .01, **P < .001 vs no inhibitor treated group in panels C-F.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/13/10.1182_blood-2016-09-739334/4/m_blood739334f2.jpeg?Expires=1768302734&Signature=D4HUbAexLSfnEVrH3nbCwF9AZNMn9YpLWeMHsF~Z7u~vUZ5Td0s1wyeur0gy8ul0O9b~N6ZpsAOvb2uUN51681XIpc3rgap942Oqr9ncgK5BjOZu0AEKHd8bT~K9As0XiNTBfG7sQOiyRdgloECCuVd5A4UXrYJRgkIKfLxu4yDenMg2Gnk9xpx7~77l6-~ONK1~pLR962Mh-W35qPh-0GQe7KJ0YZMHFuGSPDOZMBWi~~ogl-kqu1PMvSnGjj8h9qZOMkclgdfTNVwOaYy2WeufXE0eOVSCb0acGEP62QocqDEDgMHcK2C9bvNh5MeaUmJi--usLMDgbaNS9pxjPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Effect of ATRA on integrated PCA of cytokine-primed APL cells. Cytokine-primed APL cells were treated with or without ATRA for the indicated time and then incubated with MDP from healthy controls. (A) Coagulation time was measured using a recalcification-time assay. (B) Fibrin production was measured by turbidity at 405 nm (optical density 405 [OD405]). (C-D) For inhibition assays, ATRA-differentiated APL cells were treated with DNase I, anti-TF antibody, or lactadherin before incubation with plasma. Coagulation time and fibrin formation were then evaluated. (E-F) Inhibition assays were also performed using APL cells treated with PBS as a control. Data are representative of 4 independent experiments and are displayed as mean ± SD. *P < .05, **P < .01 vs day 0; #P < .05, ##P < .01 vs ATRA-treated group in panels A-B; *P < .01, **P < .001 vs no inhibitor treated group in panels C-F.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/13/10.1182_blood-2016-09-739334/4/m_blood739334f2.jpeg?Expires=1768423102&Signature=eb~keHH0HUQ6awM2sqbZ84rwsb28je5Lo1nT7qXaVZaje1Rl4jNnyw5EE3PEQ7FHEWvq1Dt4kg17QSgphiphJIx-Qn5tIUohi8pM-PQZR7P9opALiUJimSvKfm9W4DnaoQd1Cxl38w6hbi3e3xutLLkeaNkC5PA4itkonOKH87Nsg1ed~G0z1I3cPiCB1FZQs1-x~dANS5wGbQ~on2Mpc5xw0mdOu8l8G9hR4SgXI1BInQBVAsIPQS1nTXcVabEAWyU~5m7jYmrkX-YXAmLs5OtpXMXTzjZ8Z1dJGHYrJ-KFh8fjmams3956WVq0YITqFx0uJxE9lE36MttoNqe1eQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)