Key Points
S100A9 induces differentiation and growth arrest of AML cells via TLR4.
S100A8 regulates S100A9 activity and sustains AML immature phenotype.
Abstract
S100A8 and S100A9 are calcium-binding proteins predominantly expressed by neutrophils and monocytes and play key roles in both normal and pathological inflammation. Recently, both proteins were found to promote tumor progression through the establishment of premetastatic niches and inhibit antitumor immune responses. Although S100A8 and S100A9 have been studied in solid cancers, their functions in hematological malignancies remain poorly understood. However, S100A8 and S100A9 are highly expressed in acute myeloid leukemia (AML), and S100A8 expression has been linked to poor prognosis in AML. We identified a small subpopulation of cells expressing S100A8 and S100A9 in AML mouse models and primary human AML samples. In vitro and in vivo analyses revealed that S100A9 induces AML cell differentiation, whereas S100A8 prevents differentiation induced by S100A9 activity and maintains AML immature phenotype. Treatment with recombinant S100A9 proteins increased AML cell maturation, induced growth arrest, and prolonged survival in an AML mouse model. Interestingly, anti-S100A8 antibody treatment had effects similar to those of S100A9 therapy in vivo, suggesting that high ratios of S100A9 over S100A8 are required to induce differentiation. Our in vitro studies on the mechanisms/pathways involved in leukemic cell differentiation revealed that binding of S100A9 to Toll-like receptor 4 (TLR4) promotes activation of p38 mitogen-activated protein kinase, extracellular signal-regulated kinases 1 and 2, and Jun N-terminal kinase signaling pathways, leading to myelomonocytic and monocytic AML cell differentiation. These findings indicate that S100A8 and S100A9 are regulators of myeloid differentiation in leukemia and have therapeutic potential in myelomonocytic and monocytic AMLs.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous clonal disorder arising from the acquisition of genetic and/or epigenetic lesions by stem or progenitor hematopoietic cells. Accumulation of these abnormalities results in disruption of differentiation and increased proliferation, leading to blast-cell buildup in the bone marrow (BM) and other tissues. Currently, the standard therapy approach for AML is induction chemotherapy using a combination of cytotoxic agents, cytarabine and anthracyclines, aimed at killing highly proliferating cells. Despite these treatments (which are associated with toxicity and morbidity), the clinical outcome remains problematic, with an abysmal 5-year survival rate for patients age >65 years (<10% survival).1,2 The high rates of relapse and toxicity associated with treatments also highlight the need for new therapeutic options. Today, the only effective targeted therapy for AML is the induction of differentiation by all-trans-retinoic acid in acute promyelocytic leukemia.3 This indicates that differentiation therapy could be beneficial to patients with AML, particularly in subtypes displaying more mature phenotypes.
S100A8 and S100A9, also known as myeloid-related proteins, are members of the S100 family of calcium-binding proteins. These cytoplasmic proteins are abundantly expressed in myeloid cells, representing up to 40% and 5% of cytosolic proteins in neutrophils and monocytes, respectively.4 S100A8 and S100A9 expression is modulated during myelopoiesis, with expression at low levels in myeloid progenitor cells and upregulated during granulocyte and monocyte differentiation.5-8 S100A8 and S100A9 exist as homodimers and associate to form the heterodimer S100A8/A9, known as calprotectin. These dimers are biologically active and could exert distinct intracellular and extracellular functions. Once secreted, S100A8 and S100A9 induce immune and inflammatory responses9 through interaction with receptors such as Toll-like receptor 4 (TLR4), receptor for advanced glycation end-product (RAGE), and CD33.10-12 S100A8 and S100A9 are strongly upregulated in many tumors, including prostatic, colon, pancreatic, breast, and skin cancers, where they stimulate the recruitment of myeloid cells, as well as myeloid-derived suppressor cells, leading to tumor growth, formation of premetastatic niche, and metastasis.13-18 Gene expression studies identified S100A8 and S100A9 as two of the most overexpressed genes in subgroups of AMLs.19 Further, expression of S100A8 is a predictor of poor survival in AML.20
The potential functions of S100A8 and S100A9 in leukemogenesis remain unknown. Hence, we investigated the biological roles of S100A8 and S100A9 in a myelomonocytic AML mouse model and human models of AML, as well as in primary human AML samples. We identified S100A9 as a potential differentiation agent in myelomonocytic and monocytic AMLs.
Material and methods
Mice
All experiments were carried out in accordance with the Université Laval Animal Protection Committee (Quebec City, QC, Canada). Female donor B6.SJL (CD45.1) or C57BL/6 (CD45.2) and female recipient C57BL/6 (CD45.2) mice were obtained from Jackson Laboratories and used at 6 to 12 weeks old. S100A9-knockout (S100A9KO) mice were generated by the National Institutes of Health Knockout Mouse Project and bred at Université Laval, Quebec, Canada. S100A8-knockout (S100A8KO) mice were generated by Lexicon Pharmaceuticals, Inc., by deletion of all coding exons of S100A8 gene. S100A8KO and S100A9KO mice (mixed 129/SvEvBrd and C57BL/6J genetic background) were backcrossed to C57/BL6 background (Jackson Laboratories) for 10 generations. S100A8KO mice were viable, fertile, and phenotypically normal.
Transduction of murine primary BM progenitor cells
BM cells were harvested from 5-fluorouracil–treated (150 mg/kg) C57BL/6 wild-type (WT) or S100A8KO mice (8- to 12-week-old females) and then stimulated as previously described.21 Cells were cocultured for 2 days with irradiated (150 Gy) virus-producer GP+E86 cells expressing murine stem-cell virus (MSCV)-hoxa9-IRES-meis1a-hpgk-EGFP (mpgk-Neo cassette from the vector, kindly provided by G. Sauvageau, Montreal, Canada, was replaced with hpgk-EGFP), MSCV-MLL-ENL-hpgk-EGFP, or control MSCV-EGFP in the presence of polybrene (6 μg/mL; Sigma-Aldrich) as previously described.22
BM transplantation
Irradiated (7 Gy) C57BL/6 WT or S100A8KO recipient mice were intravenously injected with 4 × 105 infected murine BM cells. For secondary transplantation, 2 × 105 BM cells from primary leukemia were injected into sublethally irradiated (4.5 Gy) recipients via the tail vein.
Recombinant proteins and antibody production
Recombinant murine (rm) and human (rh) S100A8 and S100A9 as well as anti-S100A9 monoclonal antibodies (mAbs) and anti-S100A8 polyclonal antibodies (pAbs) were produced as described previously.23-26 Specificity of the murine antibodies was confirmed by enzyme-linked immunosorbent assay (ELISA). Antibodies used in all in vivo experiments only recognized either murine S100A8 or S100A9.
In vivo treatment of AML mice
All treatments were performed using Hoxa9+Meis1 secondary recipient mice. For antibody treatment, mice received 10 mg/kg of body weight of pAb anti-S100A8 or mAb anti-S100A9 or control immunoglobulin G (IgG) intraperitoneally (i.p.) 3 times per week starting on day 4 posttransplantation until mice were moribund. For protein treatment, recipient mice were injected i.p. 3 times per week with 20 µg/mouse rmS100A8 or rmS100A9 or phosphate buffer saline starting on day 4 posttransplantation. For comparative studies between induction chemotherapy and protein and as antibody treatments, all injections were started at day 15 posttransplantation when blasts in peripheral blood reached 5%. Chemotherapy was given as follows: cytarabine (100 mg/kg; Hospira) was administrated i.p. every day for 5 days; doxorubicin (Adriamycin; 3 mg/kg; Pfizer) was administrated i.p. every day for the first 3 days. Mice received rmS100A9 (20 µg/mouse) or pAb anti-S100A8 (10 mg/kg) i.p. 3 times per week until mice were moribund.
Measurement of S100A8 and S100A9 levels in plasma
CB-cell culture and transduction
Cord blood (CB) samples were obtained according to procedures approved by the ethics research committees of the Centre Hospitalier Universitaire (CHU) de Québec and Hotel-Dieu de Lévis. CD34+ CB cells were isolated by negative selection (EasySep Human Progenitor Enrichment Cocktail; Stem Cell Technologies) and then transduced with retrovirus-expressing MSCV-MLL-AF9-hpgk-EGFP as previously described.22
In vitro differentiation assays
Cells were plated in duplicates in 96-well plates (2 × 105 cells/well) and stimulated with 20-µg/mL rhS100A9 or rhS100A8. Cells were preincubated for 2 hours with extracellular signal-regulated kinase (ERK) inhibitor U0126 (10 µM), Jun N-terminal kinase (JNK) inhibitor SP600125 (10 µM), p38 MAPK inhibitor SB203580 (10 µM), or Akt inhibitor MK2206 (10 µM). After 48 hours, cells were collected, and differentiation was determined by flow cytometry.
AML specimen
AML cell samples used in this study were collected by the Banque de Cellules Leucémiques du Québec with informed consent and project approval by the research ethics board of the Maisonneuve-Rosemont Hospital. AML cells were cultivated as described previously.28 For differentiation assays, cells were seeded in 24-well plates with 20 µg/mL rhS100A9 for 48 hours.
Cell-cycle analysis
Cell cycle was analyzed by incorporating propidium iodide. Leukemic cells from BM, spleen, and blood of S100A9-treated mice were washed twice and fixed in ethanol overnight. Cells were resuspended in 5 µg/mL ribonuclease A (Sigma-Aldrich) and stained with propidium iodide (5 µg/mL; Sigma-Aldrich) for 15 minutes.
Flow cytometry analysis
Single-cell suspensions of BM, spleen, and blood cells were stained for 30 minutes on ice with various antibodies (all antibodies were obtained from BD Biosciences unless otherwise stated): CD11b-PerCPCy5.5 (clone M1/70), Gr1-APC (RB6 8C5), Ly6G-APC (1A8), c-kit-APC-H7 (2B8), and TLR4-PECy7 (SA15-21). Staining of human AML cells was performed as described for murine cells with the following antibodies: CD11b-PE-Cy5 (Bear1; Beckman Coulter), CD14-PE (RM052; Beckman Coulter), TLR4-APC (HTA125; eBioscience), and CD33-APC (P67.6; BD Biosciences). Intracellular staining of S100A8 and S100A9 on human or murine AML cells was performed with polyclonal rabbit anti-S100A8 or anti-S100A9 IgG for 30 minutes. Cells were then incubated with Alexa647 goat anti-IgG rabbit (Life Technologies). Acquisition was performed on FACSCanto (BD Biosciences), and results were analyzed using FlowJo software (TreeStar, Inc.).
Metabolic assays
Real-time analyses of the extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) were performed using an XFe-96 Extracellular Flux Analyzer (Seahorse Bioscience). After treatment, cells were seeded in XF cell plates (2 × 105 cells/well) and incubated in unbuffered medium for 1 hour before being assayed. Measurements of OCR and ECAR were performed as described previously.29 OCR/ECAR ratio was determined from basal levels of glycolysis and mitochondrial respiration.
Multiplex phosphoprotein assay
Mono-Mac-1 cells (kindly provided by M. J. Tremblay, Quebec, Canada) were serum-starved over 12 to 16 hours. Cells were then incubated in serum-free medium with 20 µg/mL rhS100A9 at 37°C. At the indicated times, cells were lysed using Bio-Plex cell lysis buffer (Bio-Rad). Phosphoprotein levels were measured using the Bio-Plex Pro cell-signaling assay (Bio-Rad) according to the manufacturer’s instructions. The analyzed phosphoproteins were Akt (Ser473), c-Jun (Ser63), ERK1/2 (Thr202/Tyr204, Thr185/Tyr187), JNK (Thr183/Tyr185), p38 MAPK (Thr180/Tyr182), CREB (Ser133), mTOR (Ser2448), and NFκB-p65 (Ser536). Results were normalized to glyceraldehyde-3-phosphate dehydrogenase.
Data analysis
Statistical analyses were performed using Prism 6 software (GraphPad Software, Inc.). Data are expressed as the mean ± standard error of the mean. Statistical analysis was performed using the 2-tailed Student t test, assuming equal sample variance. Statistical analysis by Kaplan-Meier survival curve was performed using the Gehan-Breslow-Wilcoxon and Mantel-Cox tests to compare 2 groups. The results were considered statistically significant at P value < .05, < .01, < .001, or < .0001.
Results
Subpopulation of leukemic cells secretes high levels of S100A8 and S100A9 proteins in myelomonocytic and monocytic AMLs
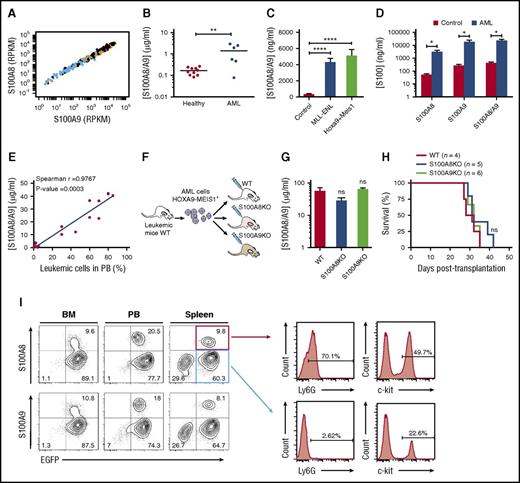
We first confirmed expression of S100A8 and S100A9 from RNA-sequencing data collected from 437 human AML samples (Leucegene Project; www.leucegene.ca). As reported in The Cancer Genome Atlas, high expression of S100A8 and S100A9 was found in myelomonocytic and monocytic AMLs (French-American-British M4 and M5) compared with poorly differentiated AML (French-American-British M0 and M1, blue dots; Figure 1A).19 Elevated S100A8/A9 levels were also found in plasma of patients with AML compared with healthy individuals (Figure 1B). Mice with AML induced by overexpression of Hoxa9 and cofactor Meis1 (H9M1) or expressing fusion gene MLL-ENL in mouse stem/progenitor cells displayed higher levels of S100A8/A9 than controls, indicating that the proteins are secreted (Figure 1C). Although the heterodimeric form was predominant, homodimeric S100A8 and S100A9 plasmatic levels were also increased in the AML models (Figure 1D). S100A8/A9 concentrations gradually increased as leukemia progressed and strongly correlated with mobilization of leukemic cells from the BM to the peripheral blood (Figure 1E), reflecting disease progression.
Expression of S100A8 and S100A9 in AML. (A) S100A8 and S100A9 RNA expression in patients with AML in the Leucegene cohort. Each dot represents 1 patient: orange, myelomonocytic and monocytic AML; blue, undifferentiated M0 and M1 AML. (B) S100A8/A9 concentration in plasma of patients with AML (n = 6) and healthy controls (n = 10) measured by ELISA. (C) S100A8/A9 plasma concentrations in control and MLL-ENL or H9M1 AML mice (control, n = 5; AML, n = 3). (D) Concentrations of heterodimeric S100A8/A9 and homodimeric S100A8 and S100A9 in plasma of control or H9M1 AML mice at death (n = 3). (E) Correlation of plasma S100A8/A9 concentration with percentage of leukemic cells in peripheral blood (PB) of H9M1 secondary recipients during leukemia progression. (F) Schematic representation of the in vivo experiments. (G) S100A8/A9 concentrations in plasma at death of WT, S100A8KO, and S100A9KO recipient mice receiving WT AML cell transplants (n = 6). (H) Kaplan-Meier survival curves of mice (WT, S100A8KO, or S100A9KO) receiving 2 × 106 BM cell transplants from WT C57BL/6-expressing H9M1. (I) Intracellular flow cytometry analyses of S100A8 and S100A9 expression in BM, PB, and spleen of H9M1 AML mice (left). Ly6G and c-kit marker expression in AML S100A+ and S100A− subpopulations (right). Data are represented as the mean ± standard error of the mean. Two-tailed Student t tests were used to assess statistical significance from 2 independent experiments. *P < .05; **P < .01; ****P < .0001. ns, not significant; RPKM, reads per kilobase of transcript per million.
Expression of S100A8 and S100A9 in AML. (A) S100A8 and S100A9 RNA expression in patients with AML in the Leucegene cohort. Each dot represents 1 patient: orange, myelomonocytic and monocytic AML; blue, undifferentiated M0 and M1 AML. (B) S100A8/A9 concentration in plasma of patients with AML (n = 6) and healthy controls (n = 10) measured by ELISA. (C) S100A8/A9 plasma concentrations in control and MLL-ENL or H9M1 AML mice (control, n = 5; AML, n = 3). (D) Concentrations of heterodimeric S100A8/A9 and homodimeric S100A8 and S100A9 in plasma of control or H9M1 AML mice at death (n = 3). (E) Correlation of plasma S100A8/A9 concentration with percentage of leukemic cells in peripheral blood (PB) of H9M1 secondary recipients during leukemia progression. (F) Schematic representation of the in vivo experiments. (G) S100A8/A9 concentrations in plasma at death of WT, S100A8KO, and S100A9KO recipient mice receiving WT AML cell transplants (n = 6). (H) Kaplan-Meier survival curves of mice (WT, S100A8KO, or S100A9KO) receiving 2 × 106 BM cell transplants from WT C57BL/6-expressing H9M1. (I) Intracellular flow cytometry analyses of S100A8 and S100A9 expression in BM, PB, and spleen of H9M1 AML mice (left). Ly6G and c-kit marker expression in AML S100A+ and S100A− subpopulations (right). Data are represented as the mean ± standard error of the mean. Two-tailed Student t tests were used to assess statistical significance from 2 independent experiments. *P < .05; **P < .01; ****P < .0001. ns, not significant; RPKM, reads per kilobase of transcript per million.
To confirm S100A8 and S100A9 secretion by leukemic cells in AML serum, H9M1 leukemic cells from C57BL/6 WT mice were injected into irradiated WT, S100A8KO, or S100A9KO recipients (Figure 1F). The results indicated similar S100A8/A9 serum concentration in S100A8KO, S100A9KO, and WT recipient mice receiving WT AML cell transplants (Figure 1G), indicating that S100 proteins are secreted by leukemic blasts and not by the microenvironment. Further, all recipient mice receiving WT H9M1+ cell transplants developed disease, with similar clinical outcomes, phenotypes, and latency periods (Figure 1H; supplemental Figure 1A; supplemental Table 1 [available on the Blood Web site]).
Although a vast majority (>90%) of BM cells were leukemic at the time of euthanization, only ∼10% of cells expressed S100A8 and S100A9 (Figure 1I). S100A8+ and S100A9+ populations were also found in MLL-ENL mice with AML with a similar infiltration in all tissues tested (supplemental Figure 1B-C). Most leukemic cells (GFP+ cells) displayed the myeloid surface markers CD11b and Gr1 (Ly6C for a majority of cells), as well as c-kit in H9M1-induced AML (data not shown). High intracellular expression of S100A8 and S100A9 was observed in CD11b+, Ly6G+, and c-kit+ AML cells (Figure 1I;supplemental Figure 1D). S100A8 and S100A9 were also detected on the surface of all AML cells (supplemental Figure 1E). These results indicate that S100A8 and S100A9 are secreted by a subpopulation of AML cells, and their presence in sera and on the surface of all AML cells suggests an extracellular function.
To determine whether S100A8 and/or S100A9 are crucial to initiation of the leukemogenic process, we transduced S100A8KO stem/progenitor cells, which also do not express S100A9 protein (supplemental Figure 2A-B), with H9M1. No substantial differences in AML latency were observed (supplemental Figure 2C-D), suggesting that the S100A proteins are dispensable to leukemogenesis. However, a decrease of approximately twofold of c-kit+ population in leukemic S100A8KO cells was noted, suggesting a potential role in myeloid progenitor accumulation (supplemental Figure 2E).
Blocking S100A8 significantly impairs AML progression in vivo
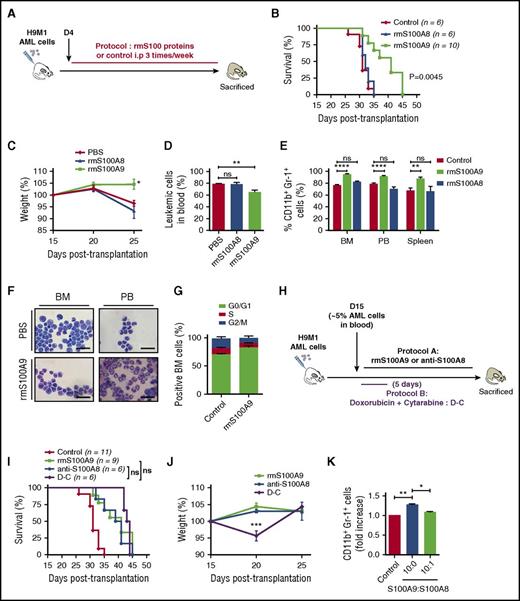
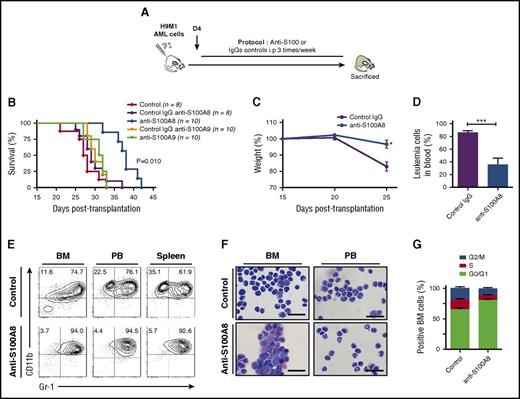
To evaluate the contribution of S100A proteins to leukemia progression, H9M1-driven AML secondary recipients were treated with antibodies against S100A8 or S100A9 (recognizing both the homodimeric and heterodimeric forms; Figure 2A; supplemental Table 2).23 Injections of anti-S100A8 significantly delayed leukemia symptoms and markedly extended survival compared with IgG (median survival: 31 days for control IgG vs 39.5 days for anti-S100A8–treated mice; P = .010; Figure 2B). However, no differences were observed with anti-S100A9 treatment (Figure 2B). Anti-S100A8 antibody–treated mice showed reduced weight loss, improved behavior, and significant reduction of AML cells in peripheral blood (86.3% ± 2.6% vs 47.6% ± 10.9%; P < .009; Figure 2B-D). Conversely, no differences were observed between control IgG and anti-S100A9–treated mice in terms of serum S100A8/A9 levels, proportion of blast cells in blood, and overall survival (Figure 2B; data not shown). Flow cytometry analysis revealed an increase of mature myeloid cell marker CD11b and Gr1 expression with anti-S100A8 treatment (Figure 2E), and the mature phenotype was confirmed by cytological observations (Figure 2F). Anti-S100A8 injections also reduced cell proliferation, which led to the accumulation of AML cells in G0/G1 cell cycle (Figure 2G). These results strongly suggest that production of S100A8 by AML cells contributes to the inhibition of cell differentiation and promotes cell proliferation, thereby contributing to leukemogenesis.
Increasing the S100A9:S100A8 ratio in vivo significantly impairs AML progression and induces cell differentiation. (A) Schematic illustration of experimental design. (B) Survival curves of secondary H9M1 AML recipients treated with pAb anti-S100A8, mAb anti-S100A9, or control IgGs at 10 mg/kg of body weight until moribund (data are from 1 of 3 independents experiments). (C) Evolution of weight during AML progression of mice treated with anti-S100A8 or control antibodies (n = 8). (D) Percentage of leukemia cells in peripheral blood (PB) in control and anti-S100A8–treated leukemic mice after 4 weeks posttransplantation. (E) Flow cytometry analyses of AML cells from representative mice treated with anti-S100A8. Contour plots are gated on EGFP+ cells. (F) Representative May-Grunwald-Giemsa–stained cytospins of AML cells from anti-S100A8–treated mice showing signs of granulocytic differentiation. Scale bars, 20 µm. (G) Cell-cycle profiles of BM cells from control and anti-S100A8 recipients (n = 5). Results are represented as the mean ± standard error of the mean. P value was determined by Student t test. *P < .05 or ***P < .001 from 3 independent experiments.
Increasing the S100A9:S100A8 ratio in vivo significantly impairs AML progression and induces cell differentiation. (A) Schematic illustration of experimental design. (B) Survival curves of secondary H9M1 AML recipients treated with pAb anti-S100A8, mAb anti-S100A9, or control IgGs at 10 mg/kg of body weight until moribund (data are from 1 of 3 independents experiments). (C) Evolution of weight during AML progression of mice treated with anti-S100A8 or control antibodies (n = 8). (D) Percentage of leukemia cells in peripheral blood (PB) in control and anti-S100A8–treated leukemic mice after 4 weeks posttransplantation. (E) Flow cytometry analyses of AML cells from representative mice treated with anti-S100A8. Contour plots are gated on EGFP+ cells. (F) Representative May-Grunwald-Giemsa–stained cytospins of AML cells from anti-S100A8–treated mice showing signs of granulocytic differentiation. Scale bars, 20 µm. (G) Cell-cycle profiles of BM cells from control and anti-S100A8 recipients (n = 5). Results are represented as the mean ± standard error of the mean. P value was determined by Student t test. *P < .05 or ***P < .001 from 3 independent experiments.
S100A9 induces cellular differentiation and prolongs survival of leukemic mice
To test whether S100A8 and S100A9 proteins could regulate the differentiation of AML cells, WT H9M1 secondary recipient mice were treated with phosphate buffer saline or rmS100A8 or rmS100A9 proteins (Figure 3A; supplemental Table 2). Surprisingly, treatment with rmS100A8 had no effect on survival, whereas rmS100A9 diminished symptoms and weight loss, improved behavior, and significantly prolonged survival of AML mice (median survival: 32 days for control vs 41 days for S100A9-treated mice; P = .0045) compared with control mice (Figure 3B-D). Remarkably, 2 of 40 mice treated with rmS100A9 on four different protocols were completely cleared of leukemia (supplemental Figure 3A). As observed previously with anti-S100A8 treatment, rmS100A9 induced a marked increase of mature myeloid cells and reduced cell proliferation (Figure 3E-G). By combining injections of S100A9 and anti-S100A8, we observed a modest, but not statistically significant, increase in survival (supplemental Figure 3B-C). Interestingly, the survival rate of mice treated with either rmS100A9 or anti-S100A8 was comparable to that with standard induction chemotherapy (median survival: 41 days for rmS100A9 and 40 days for anti-S100A8 vs 43 days for chemotherapy), without any toxicity, reflected in part by no weight loss (Figure 3H-J).
Increasing S100A9 levels in vivo prolongs survival and promotes maturation of leukemic cells. (A) Overview of experimental design. (B) Kaplan-Meyer survival analysis of AML secondary recipient mice treated with 20 µg/mouse rmS100A8, rmS100A9, or PBS 3 times per week until moribund. (C) Weight evolution of AML recipients treated with rmS100A8, rmS100A9, or phosphate buffer saline (PBS; n = 9 for each group). (D) Leukemic cells in peripheral blood (PB) of control and rmS100A9-treated mice during AML progression after 4 weeks posttransplantation (n = 4). (E) CD11b+Gr-1+ AML population in BM, PB, and spleen in mice untreated or treated with rmS100A9 (n = 6). (F) Representative May-Grunwald-Giemsa–stained cytospins of AML cells from rmS100A9-treated mice showing signs of granulocytic differentiation. Scale bars, 20 µm. (G) Cell-cycle profiles of BM cells from control and rmS100A9-treated recipients (n = 5). (H) Schematic of experimental design for (I) and (J). (I) Survival curves of secondary AML mice injected at day 15 with 10 mg/kg anti-S100A8 or 20 µg/mouse rmS100A9 3 times per week or treated with chemotherapy using cytarabine (100 mg/kg) for 5 days and anthracycline (doxorubicine 3 mg/kg) for 3 days. (J) Mouse weight fluctuation over time in the different treatment protocols (n = 6 per group). (K) CD11b+Gr-1+ in H9M1 leukemic cells treated with rmS100A9 in the presence or absence of rmS100A8 ex vivo. Results are represented as the mean ± standard error of the mean. P value was determined by Student t test. *P < .05, **P < .01, or ***P < .001 from 3 independent experiments. ns, not significant.
Increasing S100A9 levels in vivo prolongs survival and promotes maturation of leukemic cells. (A) Overview of experimental design. (B) Kaplan-Meyer survival analysis of AML secondary recipient mice treated with 20 µg/mouse rmS100A8, rmS100A9, or PBS 3 times per week until moribund. (C) Weight evolution of AML recipients treated with rmS100A8, rmS100A9, or phosphate buffer saline (PBS; n = 9 for each group). (D) Leukemic cells in peripheral blood (PB) of control and rmS100A9-treated mice during AML progression after 4 weeks posttransplantation (n = 4). (E) CD11b+Gr-1+ AML population in BM, PB, and spleen in mice untreated or treated with rmS100A9 (n = 6). (F) Representative May-Grunwald-Giemsa–stained cytospins of AML cells from rmS100A9-treated mice showing signs of granulocytic differentiation. Scale bars, 20 µm. (G) Cell-cycle profiles of BM cells from control and rmS100A9-treated recipients (n = 5). (H) Schematic of experimental design for (I) and (J). (I) Survival curves of secondary AML mice injected at day 15 with 10 mg/kg anti-S100A8 or 20 µg/mouse rmS100A9 3 times per week or treated with chemotherapy using cytarabine (100 mg/kg) for 5 days and anthracycline (doxorubicine 3 mg/kg) for 3 days. (J) Mouse weight fluctuation over time in the different treatment protocols (n = 6 per group). (K) CD11b+Gr-1+ in H9M1 leukemic cells treated with rmS100A9 in the presence or absence of rmS100A8 ex vivo. Results are represented as the mean ± standard error of the mean. P value was determined by Student t test. *P < .05, **P < .01, or ***P < .001 from 3 independent experiments. ns, not significant.
The in vivo data pointed to potential antagonistic effects of S100A8 and S100A9. Therefore, we tested this hypothesis by culturing H9M1 AML cells ex vivo in the presence of rmS100A8 and rmS100A9. We observed increased expression of CD11b and Gr1 with addition of rmS100A9, indicating rmS100A9-induced differentiation of AML cells. However, the addition of rmS100A8, even at low concentration (10-fold lower than S100A9), blocked the cellular differentiation induced by rmS100A9 (Figure 3K). These data suggest that S100A8 inhibits the effect of S100A9 and that increasing the ratio of S100A9 over S100A8 (S100A9 > S100A8) in murine leukemia triggers AML cell differentiation.
S100A9 induces terminal differentiation in vitro
To gain further insight into the mechanisms underlying the effects of S100A8 and S100A9 proteins on leukemia, human CB CD34+ cells were transduced with MLL-AF9 oncogene and cultured in medium promoting myeloid development.22 MLL-AF9+ cells were then stimulated with rhS100A8 or rhS100A9. Although rhS100A8 had no effect, rhS100A9 stimulated the expression of CD14 and induced marked morphological changes similar to terminal differentiation of macrophages concomitantly to growth arrest of MLL-AF9+ cells (Figure 4A-D). In addition, increased reduction of nitroblue tetrazolium was noted in S100A9-treated cells, indicating an enhanced ability of cells to generate a respiratory burst, which is a hallmark of differentiated cells (Figure 4E). Moreover, treatment with rhS100A9 induced metabolic reprogramming of AML cells, as evidenced by a reduced OCR/ECAR ratio (Figure 4F), as well as reduced basal and maximal respiration (supplemental Figure 4A-B). Overall, these findings demonstrate that S100A9 induced metabolic reprograming and terminal differentiation of human AML cells.
S100A9 promotes terminal differentiation of human AML cells through TLR4. (A) Effect of increasing doses of rhS100A8 and rhS100A9 on expression of CD14 in human CB CD34+ cells transduced with MLL-AF9. Expression of the surface marker CD14 was evaluated by flow cytometry on transduced cells (EGFP+) after stimulation with different doses of rhS100A8 or rhS100A9 for 48 hours. (B) Proliferation of human MLL-AF9+ cells stimulated with 20 µg of rhS100A9 or phosphate buffer saline (PBS) in vitro for 5 days. (C) Kinetics of CD14 expression after stimulation of human MLL-AF9+ cells with 20 µg of rhS100A9. (D) Cytospins of human MLL-AF9+ AML at different time points showing signs of macrophage maturation induced by rhS100A9 (20 µg/ml). Scale bars, 20 µm. (E) Cytospins of nitroblue tetrazolium (NBT)–positive cells counterstained with safranin 0.15% and percentages of NBT-positive cells. Scale bars, 20 µm. (F) OCR/ECAR ratio indicative of bioenergetic profile of human MLL-AF9+ AML cells stimulated with rhS100A9. Data are represented as the mean ± standard error of the mean. P value was determined by Student t test. *P < .05, **P < .01, or ***P < .001 from 3 independent experiments.
S100A9 promotes terminal differentiation of human AML cells through TLR4. (A) Effect of increasing doses of rhS100A8 and rhS100A9 on expression of CD14 in human CB CD34+ cells transduced with MLL-AF9. Expression of the surface marker CD14 was evaluated by flow cytometry on transduced cells (EGFP+) after stimulation with different doses of rhS100A8 or rhS100A9 for 48 hours. (B) Proliferation of human MLL-AF9+ cells stimulated with 20 µg of rhS100A9 or phosphate buffer saline (PBS) in vitro for 5 days. (C) Kinetics of CD14 expression after stimulation of human MLL-AF9+ cells with 20 µg of rhS100A9. (D) Cytospins of human MLL-AF9+ AML at different time points showing signs of macrophage maturation induced by rhS100A9 (20 µg/ml). Scale bars, 20 µm. (E) Cytospins of nitroblue tetrazolium (NBT)–positive cells counterstained with safranin 0.15% and percentages of NBT-positive cells. Scale bars, 20 µm. (F) OCR/ECAR ratio indicative of bioenergetic profile of human MLL-AF9+ AML cells stimulated with rhS100A9. Data are represented as the mean ± standard error of the mean. P value was determined by Student t test. *P < .05, **P < .01, or ***P < .001 from 3 independent experiments.
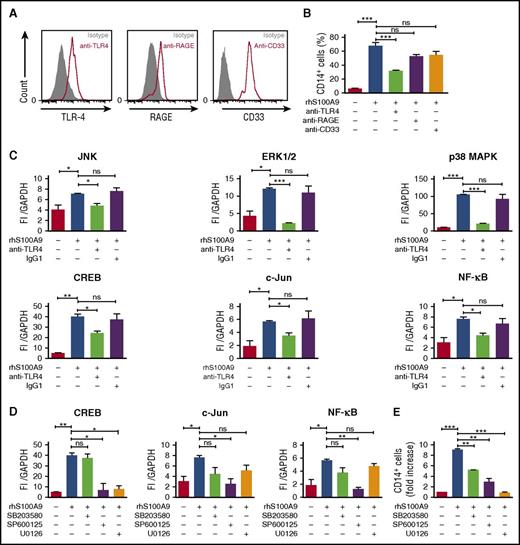
S100A9 activates ERK1/2 and JNK signaling pathways through TLR4 in human leukemic cells
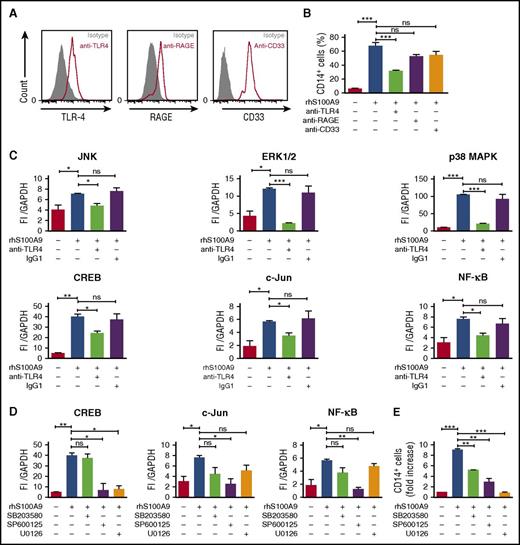
RAGE and TLR4 are well-characterized S100A8 and S100A9 receptors and expressed in AML cells (Figure 5A).10,12,15,30,31 To identify the receptor involved in S100A9-induced AML cell differentiation, MLL-AF9+ AML cells were cultured with rhS100A9 in the presence of anti-TLR4, anti-RAGE, and anti-CD33 antibodies. Neutralizing anti-TLR4 antibody markedly diminished CD14 expression after stimulation with rhS100A9 (Figure 5B), indicating that S100A9 promotes AML cell differentiation mainly through TLR4.
S100A9 activates AML cell differentiation through TLR4, ERK1/2, and c-Jun. (A) TLR4, RAGE, and CD33 expression in human MLL-AF9+ cells assessed by flow cytometry. (B) CD14 expression in human MLL-AF9+ AML cells. Cells were pretreated with neutralizing human anti-TLR4, human anti-RAGE, or human anti-CD33 (n = 5) and stimulated with rhS100A9 (20 µg/ml) for 48 hours. (C) Phosphorylation of JNK, ERK1/2, p38 MAPK, and transcription factors CREB, c-Jun, and NF-ĸB in Mono-Mac-1 cells (MLL-AF9+) treated with rhS100A9 (20 µg/ml) in the presence or absence of antibodies against TLR4. (D) Effect of inhibitors of p38 MAPK (SB352080), JNK (SP600125), and ERK1/2 (U0126) on phosphorylation of transcription factors CREB, NF-ĸB, and c-Jun. (E) CD14 expression in Mono-Mac-1 cells after stimulation with rhS100A9 (20 µg/ml) for 48 hours in the presence of SB352080 (p38 MAPK inhibitor), SP600125 (JNK inhibitor), or U0126 (ERK1/2 inhibitor). Data are expressed as the mean ± standard error of the mean. P value was determined by Student t test. *P < .05, **P < .01, or ***P < .001 from 3 independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ns, not significant.
S100A9 activates AML cell differentiation through TLR4, ERK1/2, and c-Jun. (A) TLR4, RAGE, and CD33 expression in human MLL-AF9+ cells assessed by flow cytometry. (B) CD14 expression in human MLL-AF9+ AML cells. Cells were pretreated with neutralizing human anti-TLR4, human anti-RAGE, or human anti-CD33 (n = 5) and stimulated with rhS100A9 (20 µg/ml) for 48 hours. (C) Phosphorylation of JNK, ERK1/2, p38 MAPK, and transcription factors CREB, c-Jun, and NF-ĸB in Mono-Mac-1 cells (MLL-AF9+) treated with rhS100A9 (20 µg/ml) in the presence or absence of antibodies against TLR4. (D) Effect of inhibitors of p38 MAPK (SB352080), JNK (SP600125), and ERK1/2 (U0126) on phosphorylation of transcription factors CREB, NF-ĸB, and c-Jun. (E) CD14 expression in Mono-Mac-1 cells after stimulation with rhS100A9 (20 µg/ml) for 48 hours in the presence of SB352080 (p38 MAPK inhibitor), SP600125 (JNK inhibitor), or U0126 (ERK1/2 inhibitor). Data are expressed as the mean ± standard error of the mean. P value was determined by Student t test. *P < .05, **P < .01, or ***P < .001 from 3 independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ns, not significant.
To elucidate the intracellular pathways involved in S100A9-induced differentiation, we performed a targeted multiplex assay on Mono-Mac-1 cells after incubation with rhS100A9.32,33 Addition of rhS100A9 increased the phosphorylation of p38 MAPK, ERK, Akt, and JNK (supplemental Figure 5A-B), as well as of that of transcription factors NF-κB, c-Jun, and CREB (supplemental Figure 5A). As expected, antibodies against TLR4 prevented phosphorylation induced by S100A9 (Figure 5C). Further, phosphorylation of CREB, NF-κB, and c-Jun was inhibited by SP600125 and U0126, inhibitors of JNK and ERK1/2, respectively (Figure 5D). Cell differentiation induced by S100A9 was strongly inhibited by JNK and ERK1/2 inhibitors SP600125 and U0126, respectively, whereas an attenuation was noted in the presence of p38 MAPK inhibitor SB203580 (Figure 5E). However, Akt inhibition by MK-2206 had no effect on S100A9-induced differentiation (supplemental Figure 5C). Collectively, these findings suggest that S100A9 induces AML differentiation through TLR4 and demonstrate the importance of ERK1/2 and JNK signaling pathways in this process.
S100A9 induces differentiation of cells from patients with myelomonocytic and monocytic AMLs
RNA-sequencing and flow cytometry analyses of primary human AML cells indicated that S100A8, S100A9, and TLR4 are more expressed in myelomonocytic and monocytic AMLs (orange dots) than in undifferentiated AML cells (blue dots), suggesting that the former could be more sensitive to S100A9 stimulation (Figure 6A-B; expression in genetic subgroups is presented in supplemental Figure 6). A subpopulation of S100A8+ and S100A9+ cells was present in primary myelomonocytic AML, as previously observed in murine leukemia (Figure 6C; supplemental Figure 7). Because primary AML cells are difficult to maintain in culture, the effect of S100A9 on differentiation was only tested in vitro for 48 hours using our best culture conditions.28 All 10 undifferentiated AML samples (M0-M1) were insensitive to S100A9 (Figure 6D-E; supplemental Table 3), but incubation of myelomonocytic and monocytic AML primary cells with S100A9 led to an increase in CD11b+CD14+ cells (Figure 6D-E), corroborating the differentiation observed with murine AML in vivo and human MLL-AF9+ cells.
S100A9 induces differentiation of primary human AML cells. (A) RNA expression of TLR4, S100A8, and S100A9 in primary human AML samples in the Leucegene cohort. Each dot represents 1 patient: orange, myelomonocytic and monocytic AMLs (French-American-British [FAB] M4 and M5); blue, undifferentiated AML (FAB M0 and M1). (B) Flow cytometry analysis of TLR4 expression on primary AML cells. Top 3, M0 and M1; bottom 3, M4 and M5. (C) Intracellular staining of S100A8 and S100A9 in primary human AML cells evaluated by flow cytometry. (D) Flow cytometry analysis of myeloid cell surface markers CD11b and CD14 of primary human AML cells, M0 and M1 vs M4 and M5, stimulated with rhS100A9 (20 µg/ml) for 48 hours. (E) CD11b+CD14+ cells in primary human cells from FAB M0 and M1 (left) and FAB M4 and M5 (right) patients after S100A9 stimulation (n = 10 per group). Data are expressed as the mean ± standard error of the mean. *P < .05. ns, not significant.
S100A9 induces differentiation of primary human AML cells. (A) RNA expression of TLR4, S100A8, and S100A9 in primary human AML samples in the Leucegene cohort. Each dot represents 1 patient: orange, myelomonocytic and monocytic AMLs (French-American-British [FAB] M4 and M5); blue, undifferentiated AML (FAB M0 and M1). (B) Flow cytometry analysis of TLR4 expression on primary AML cells. Top 3, M0 and M1; bottom 3, M4 and M5. (C) Intracellular staining of S100A8 and S100A9 in primary human AML cells evaluated by flow cytometry. (D) Flow cytometry analysis of myeloid cell surface markers CD11b and CD14 of primary human AML cells, M0 and M1 vs M4 and M5, stimulated with rhS100A9 (20 µg/ml) for 48 hours. (E) CD11b+CD14+ cells in primary human cells from FAB M0 and M1 (left) and FAB M4 and M5 (right) patients after S100A9 stimulation (n = 10 per group). Data are expressed as the mean ± standard error of the mean. *P < .05. ns, not significant.
Discussion
Despite a better understanding of AML pathogenesis, therapy remains unchanged, and the overall 5-year survival rate is <40%.2 The only effective targeted therapy in AML is the induction of differentiation by all-trans-retinoic acid in acute promyelocytic leukemia, which cures up to 90% of patients.34 The present study demonstrates that S100A8 and S100A9, both produced by AML cells, regulate the differentiation and proliferation of AML cells. Blocking of S100A8 or addition of S100A9 induced the differentiation of AML cells and their growth arrest in mouse and human models of AML in primary cells from patients with AML. S100A9 activates TLR4, leading to the phosphorylation of p38 MAPK, ERK1/2, and JNK, which in turn activate CREB, c-JUN, and NF-κB. Injection of S100A9 proteins or anti-S100A8 antibodies was nearly as effective as conventional chemotherapy in prolonging survival of AML mice without serious adverse effects.
Concentrations of S100A8/A9 are elevated in the plasma of patients with AML, and a small population of leukemic cells expressing S100A8 and S100A9 was consistently observed in H9M1 and MLL-ENL mouse models, as well as in primary human AML cells. This subpopulation (displaying the phenotype CD11b+Ly6G+c-kit+) is the probable origin of the high plasmatic concentrations of hetero- and homodimers of S100A8 and S100A9. In addition, expression of TLR4 is increased in these cells (data not shown), suggesting an autocrine loop. Interestingly, a study comparing 2 engineered murine H9M1 AMLs with different leukemic stem-cell (LSC) frequencies (1 in 1.4 vs 1 in 347) identified S100A8 and S100A9 as the most upregulated genes in high–LSC frequency AML.35 Through a limit dilution assay analysis, we observed that the frequency of LSCs was diminished by half in S100A8KO H9M1+ AML cells (data not shown). This finding suggests that the S100A+ subpopulation may contain the LSC fraction and that S100A8 may regulate LSCs.
S100A8 and S100A9 are preferentially coexpressed as the heterodimer.31 We showed that S100A8 inhibits S100A9-induced terminal differentiation of AML cells and favors AML growth. Similarly, Schneider et al36 demonstrated that downregulation of S100A8 restored erythroid differentiation in a mouse model of myelodysplastic syndrome. In addition, preliminary observations of S100A8KO mice revealed a significant increase of myeloid cells in the BM (in particular, neutrophils, monocytes, and their precursors; data not shown), suggesting that S100A8 regulates myelopoiesis. Collectively, these data emphasize a role for S100A8 in leukemogenesis by maintaining an undifferentiated phenotype through an autocrine/paracrine pathway.
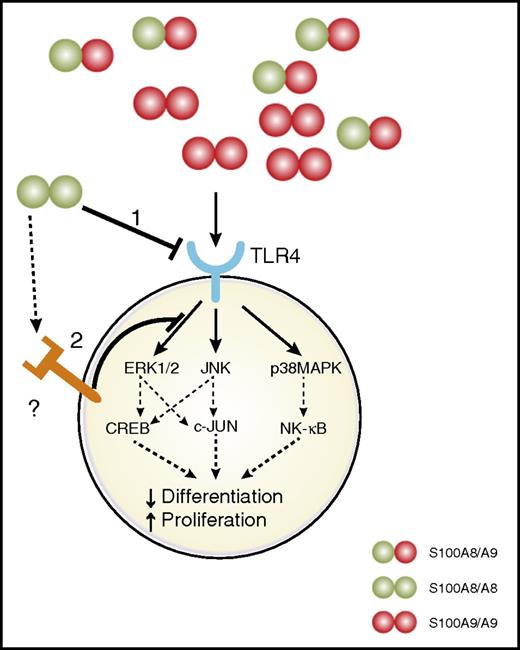
How S100A8 blocks myeloid differentiation in AML is still unknown and is an important direction for future study. Considering that a 1:10 ratio of S100A8:S100A9 is sufficient to abolish differentiation of leukemic cells, it seems unlikely that this phenomenon is the result of a competition for receptor binding or formation of the heterodimer (Figure 7). We can speculate that S100A8 acts through another receptor to antagonize TLR4 activation by S100A9 or that a higher affinity/avidity of S100A8 for TLR4 triggers other intracellular pathways. However, the latter hypothesis seems less likely, because multiplex assays failed to reveal phosphorylation induced by S100A8. In addition, S100A9 has a higher affinity for TLR4 than S100A8.37 Increased degradation of S100A9 induced by binding with S100A8 is also unlikely, because the S100A8:S100A9 ratio in AML is ∼1:10, and injecting S100A8 in vivo had no effect on plasmatic S100A9 levels (data not shown).
Effects of S100A8 and S100A9 on AML cell differentiation and proliferation. S100A8 and S100A9 are secreted by a subpopulation of leukemic cells (CD11b+Ly6G+c-kit+) in AML. S100A9 binds to TLR4 and induces signaling pathways, promoting leukemic cell differentiation and proliferation arrest. (1) S100A8 could bind to TLR4 and activate different signaling pathways, leading to the inhibition of cellular differentiation induced by S100A9. (2) Alternatively, S100A8 could interact with a different receptor and induce an antagonizing signal to inhibit S100A9-induced differentiation.
Effects of S100A8 and S100A9 on AML cell differentiation and proliferation. S100A8 and S100A9 are secreted by a subpopulation of leukemic cells (CD11b+Ly6G+c-kit+) in AML. S100A9 binds to TLR4 and induces signaling pathways, promoting leukemic cell differentiation and proliferation arrest. (1) S100A8 could bind to TLR4 and activate different signaling pathways, leading to the inhibition of cellular differentiation induced by S100A9. (2) Alternatively, S100A8 could interact with a different receptor and induce an antagonizing signal to inhibit S100A9-induced differentiation.
Interestingly, in the present study, we report that extracellular S100A9 induces terminal differentiation of myeloid leukemia cells in human and murine AMLs after TLR4 activation, which is highly expressed by primary myelomonocytic and monocytic leukemia cells. Similarly, a TLR8 agonist has been shown to induce the differentiation of monocytic cell lines, suggesting the possible existence of a common effect of TLR agonists on myeloid cell differentiation.38 In contrast, anti-S100A8 induced the differentiation of AML cells, suggesting that the differentiation-promoting effect of S100A9 is inhibited by S100A8.
Binding of S100A9 to TLR4 stimulates the phosphorylation of JNK, ERK1/2, and p38 MAPK, which leads to the activation of c-Jun, CREB, and NF-κB. Activation of neutrophils by S100A9 also proceeds via p38 MAPK, JNK, and ERK1/2 phosphorylation.39 These pathways, known to be triggered by TLR stimulation, are key regulators involved in inflammatory responses, cytokine production, and myeloid differentiation.40 Among them, ERK1/2 and JNK signaling pathways are important positive regulators of both granulocytic and monocytic differentiation.41-44 Pharmacological inhibitors of p38 MAPK and JNK partially inhibited S100A9-induced CD14 expression. In contrast, inhibition of ERK1/2 phosphorylation completely abrogated the differentiation induced by S100A9, indicating that ERK1/2 is probably the principal transduction pathway leading to cell differentiation.
TLR4 is highly expressed by myelomonocytic and monocytic AMLs, and S100A9 exhibits a pronounced effect in these subtypes, which represent ∼30% of all AMLs. A differentiation therapy targeting the S100A8/S100A9-TLR4-ERK/JNK/p38 pathway could therefore have a significant impact on the clinical outcome of patients with AML. The feasibility of this therapeutic strategy is highlighted by experiments aimed at reducing S100A8 or increasing S100A9, which prolonged survival of leukemic mice to an extent comparable to that achieved with standard induction chemotherapy. Targeting these endogenous proteins showed significant benefits without adverse effects. Therefore, this study could pave the way to alternative AML therapeutic approaches aimed at cell differentiation of myelomonocytic and monocytic AMLs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the team of the Banque de Cellules Leucémiques du Québec and its director, Josée Hébert, who provided and characterized AML samples included in this study, and the team of physicians and nurses at both CHU de Québec and Hotel-Dieu de Lévis for cord blood collection.
This work was supported by grants from Fonds de Recherche du Québec-Santé (FRSQ)–Pfizer and the Merck-Dohme-Fondation de l’Université Laval. The Banque de Cellules Leucémiques du Québec is supported by a grant from the Cancer Research Network of FRQS.
Authorship
Contribution: M.L., M.R.T., P.A.T., and F.B. designed the study; M.L. performed the experiments; A.L. and M.P. performed the bioenergetic profile; M.-A.R. quantified S100A8/A9 in patients’ plasma; L.G. provided human MLL-AF9 cells; and M.L., P.A.T., and F.B. wrote the manuscript with help from the other coauthors.
Conflict-of-interest disclosure: P.A.T. is the founder of InflammatoRx, Inc., which is developing anti-S100A9 antibodies for commercial purposes. The remaining authors declare no competing financial interests.
Correspondence: Frédéric Barabé, Centre de Recherche du CHU de Québec–Université Laval, 2705 Boul Laurier local R0-709, Quebec, QC G1V 4G2, Canada; e-mail: frederic.barabe@crchudequebec.ulaval.ca; and Philippe A. Tessier, Centre de Recherche du CHU de Québec–Université Laval, 2705 Boul Laurier local R0-709, Quebec, QC G1V 4G2, Canada; e-mail: philippe.tessier@crchudequebec.ulaval.ca.



![Figure 6. S100A9 induces differentiation of primary human AML cells. (A) RNA expression of TLR4, S100A8, and S100A9 in primary human AML samples in the Leucegene cohort. Each dot represents 1 patient: orange, myelomonocytic and monocytic AMLs (French-American-British [FAB] M4 and M5); blue, undifferentiated AML (FAB M0 and M1). (B) Flow cytometry analysis of TLR4 expression on primary AML cells. Top 3, M0 and M1; bottom 3, M4 and M5. (C) Intracellular staining of S100A8 and S100A9 in primary human AML cells evaluated by flow cytometry. (D) Flow cytometry analysis of myeloid cell surface markers CD11b and CD14 of primary human AML cells, M0 and M1 vs M4 and M5, stimulated with rhS100A9 (20 µg/ml) for 48 hours. (E) CD11b+CD14+ cells in primary human cells from FAB M0 and M1 (left) and FAB M4 and M5 (right) patients after S100A9 stimulation (n = 10 per group). Data are expressed as the mean ± standard error of the mean. *P < .05. ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/14/10.1182_blood-2016-09-738005/4/m_blood738005f6.jpeg?Expires=1766017992&Signature=qW1ThVJb2Zpg7K2apa~3qKqY3UW5wzv0jzmFx3L~gc-EPP3yXwwbAFVI2vZ6CTFiFOfMJ1EU~8GMtH2AGxfI6Dagn9Kx0U4l2~tjBSYR1SQ98FCjx9CoiJGcexC7dALxlPdcv2KAB5HiI5wUcse1heozXNDOdSUNODKTpesT3ybuJc8QzMbGxdhwKCi8FNjRqOeyCq0cCwlzTyOjSW5pRHb-NTVSnC9AZ2OtPJKivVrHDYcvLd0ezGb-QvwrjwS8YxVeY7trzNnwnVyRZDtSpSVxtIEcS9~PAjpCjbwu5weGQ89mywNgt-NyUyWjoVMtKtg4DZ2FBm5hKnbeEAI4CA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Figure 6. S100A9 induces differentiation of primary human AML cells. (A) RNA expression of TLR4, S100A8, and S100A9 in primary human AML samples in the Leucegene cohort. Each dot represents 1 patient: orange, myelomonocytic and monocytic AMLs (French-American-British [FAB] M4 and M5); blue, undifferentiated AML (FAB M0 and M1). (B) Flow cytometry analysis of TLR4 expression on primary AML cells. Top 3, M0 and M1; bottom 3, M4 and M5. (C) Intracellular staining of S100A8 and S100A9 in primary human AML cells evaluated by flow cytometry. (D) Flow cytometry analysis of myeloid cell surface markers CD11b and CD14 of primary human AML cells, M0 and M1 vs M4 and M5, stimulated with rhS100A9 (20 µg/ml) for 48 hours. (E) CD11b+CD14+ cells in primary human cells from FAB M0 and M1 (left) and FAB M4 and M5 (right) patients after S100A9 stimulation (n = 10 per group). Data are expressed as the mean ± standard error of the mean. *P < .05. ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/14/10.1182_blood-2016-09-738005/4/m_blood738005f6.jpeg?Expires=1766533561&Signature=gAQYPVJl~lA9oS7dyZIRTWuMGcMlB8XUCrxTgJRdSfheW3dUKhTid-CTVjHFAjx0kmBvt~BVY-PF0U3ru71-WW7CePwWKN0r9pa0pvCn5wQCxi5YAVLFV5lUvkXxeaZZYKRCVv495RSvrkmWXG02LbYuP1WufUNCSEy~AMBy3jir4lMPdZQPoXN1Ri80ZfJF51RiIKvqI76-whlO9RQ0XmE4RKisZyfnCLmy55hyl1IwZheMBSvvAO77qrZkHNCr-mnJYYPpVwCk1AZ-7uflJGPnCeU67YTqcJuk9IhY87VUTRqJzzyExsq8V3NRizUaGBJduEhLf5XQ~hBcOKFeeQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)