Key Points
PD-1 blockade with nivolumab provides durable disease control after allo-HCT.
PD-1 blockade with nivolumab after allo-HCT is associated with 30% acute GVHD.
Abstract
Allogeneic hematopoietic cell transplantation (allo-HCT) is indicated for patients with relapsed or refractory Hodgkin lymphoma (HL). Although long-term disease control can be achieved, relapse is still frequent. The programmed cell death protein 1 (PD-1) pathway-blocking antibody nivolumab has shown substantial therapeutic activity and an acceptable safety profile in patients with relapsed or refractory HL who did not receive allo-HCT. However, PD-1 blocking strategy can increase the risk of graft-versus-host disease (GVHD) in murine models. We retrospectively assessed the efficacy and toxicity of nivolumab as a single agent in 20 HL patients relapsing after allo-HCT. GVHD occurred in 6 patients (30%) after nivolumab initiation. All 6 patients had prior history of acute GVHD. The patients with nivolumab-induced GVHD were managed by standard treatment for acute GVHD. Two patients died as a result of GVHD, 1 of progressive disease and 1 of complications related to a second allo-HCT. Overall response rate was 95%. At a median follow-up of 370 days, the 1-year progression-free survival rate was 58.2% (95% CI, 33.1%-76.7%) and the overall survival rate was 78.7% (95% CI, 52.4%-91.5%). Among 13 patients still in response, 6 received a single dose of nivolumab and 7 remain on nivolumab. Compared with standard options for this indication, our results show that nivolumab is effective with an acceptable safety profile.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is a treatment option for patients with relapsed or refractory Hodgkin lymphoma (HL),1-3 particularly in those for whom a previous autologous transplantation failed. Although long-term disease control can be achieved after allo-HCT, relapse remains frequent with the probability of progression-free survival (PFS) ranging from 20% to 60% at 3 years.4,5 Treatment options are limited for patients who relapse after allo-HCT (donor lymphocyte infusion with or without chemotherapy, brentuximab vedotin, bendamustine), with discouraging results in terms of efficacy and tolerability. Recently, early-phase clinical trials of the programmed cell death protein 1 (PD-1)–blocking antibodies nivolumab and pembrolizumab have shown substantial therapeutic activity and an acceptable safety profile in patients with relapsed or refractory HL.6,7 These data led to US Food and Drug Administration approval of nivolumab in this indication. Of note, both trials excluded patients with a previous history of allo-HCT because of concerns about reactivating graft-versus-host disease (GVHD). Indeed, an increase in GVHD lethality after PD-1 blockade therapy was described in 2 murine models.8,9 Moreover, in a phase 1b trial targeting CTLA-4 with ipilimumab in patients with various relapsed hematologic cancers after allo-HCT,10,11 4 (14%) of 28 patients (including 7 with HL) experienced GVHD. When nivolumab was approved, the US Food and Drug Administration asked health care professionals to closely observe patients after allo-HCT for early evidence of transplant-related complications and also required further study of the safety of allo-HCT after nivolumab. To date, only a few case reports have been published regarding PD-1 blockade therapy in HL patients with relapsed disease after allo-HCT.12-14 Among the 4 patients reported, none developed signs of acute GVHD (aGVHD), despite a history of chronic GVHD (cGVHD) in one of them.
The purpose of this study was to investigate the safety and the efficacy of nivolumab in patients with relapsed or refractory HL after allo-HCT.
Patients and methods
After the publication of data showing substantial therapeutic activity with an acceptable safety profile of nivolumab in relapsed and refractory HL after chemotherapy and brentuximab vedotin, Agence Nationale de Sécurité du Médicament (the French medical drug agency) authorized a named patient authorization for temporary use (ATU) program of nivolumab for this indication between March and September 2015. Briefly, named patient ATU is a procedure to make medicinal products available that are strongly assumed to be effective and safe in view of the results of clinical trials before the marketing license has been granted. In this context, we retrospectively assessed the efficacy and toxicity of nivolumab as a single agent in all patients that were granted an ATU for nivolumab and whose prior therapy included allo-HCT. Twenty patients were thereby identified through the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire and the Lymphoma Study Association network, and data were collected by performing a patient-by-patient medical record review. Before starting the treatment, in accordance with the ATU procedure, each patient was informed by the prescribing physician about the conditions of receiving exceptional access to the medicinal product, including data collection and the characteristics of the medicinal product. An information sheet was given to the patient along with verbal explanations of the details. All patients had progressive disease when nivolumab therapy was started. Per ATU recommendations, nivolumab was given at a dose of 3 mg/kg of body weight once every 2 weeks without premedication until disease progression or unacceptable toxicity as assessed by investigators. Toxicities were graded retrospectively according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Efficacy was assessed by physical examination before each infusion according to criteria described by Cheson et al15 (in 2014) after 4 injections of nivolumab or 2 months after treatment initiation in case of nivolumab interruption. We then performed a systematic assessment every 3 months in the absence of overt progressive disease. The diagnosis of GVHD was confirmed by board-certified hematologists with expertise in allo-HCT on the basis of a body of clinical, biological, radiologic, and histologic findings. The distinction between aGVHD and cGVHD was made according to the National Institutes of Health 2005 criteria.16 The severity of nivolumab-induced GVHD was graded according to standard criteria.17 The Kaplan-Meier method was used to estimate overall survival (OS) and PFS probabilities from initiation of nivolumab. All statistical analyses were performed by using the R software program (https://www.r-project.org/). The OS and PFS probabilities were computed by using the R survival package. Times from allo-HCT to nivolumab initiation were compared according to presence or absence of nivolumab-induced GVHD by using the Mann-Whitney U test with the Wilcox.test function.
Results
Patient characteristics
Main patient characteristics are provided in Table 1. Median age at time of transplant was 33 years (range, 20-51 years). The median number of previous systemic therapies was 7 (range, 4-13 previous systemic therapies), including allo-HCT. Among 6 grafts (30%) coming from unrelated donors, 3 were HLA-matched and 3 were 9/10-locus mismatched. Matched-related siblings were used for 10 patients (50%), whereas 1 patient (5%) received a graft from a familial haploidentical donor. Three patients (15%) received a cord blood graft. The best response at allo-HCT was partial response (PR) in 10 patients (50%) and complete response (CR) in 9 patients (45%). One patient received allo-HCT while disease was progressive. Allo-HCT improved response in 2 patients who converted from PR to CR at first restaging (day 100). Thirteen patients received 1 or more systemic therapies between first relapse or progression after allo-HCT and initiation of nivolumab therapy, including donor lymphocyte infusions (n = 4). Prior history of GVHD was reported in 13 patients, including 10 aGVHD (but no grade III-IV) and 3 limited cGVHD. No prior GVHD was reported in 7 patients. Four patients (20%) had mild to moderate cGVHD at nivolumab initiation per National Institutes of Health 2014 criteria.18 All 20 patients stopped receiving immune suppression for more than 4 weeks before nivolumab initiation. Median number of nivolumab injections was 8 (range, 1-36).
Management and outcome of nivolumab-induced GVHD
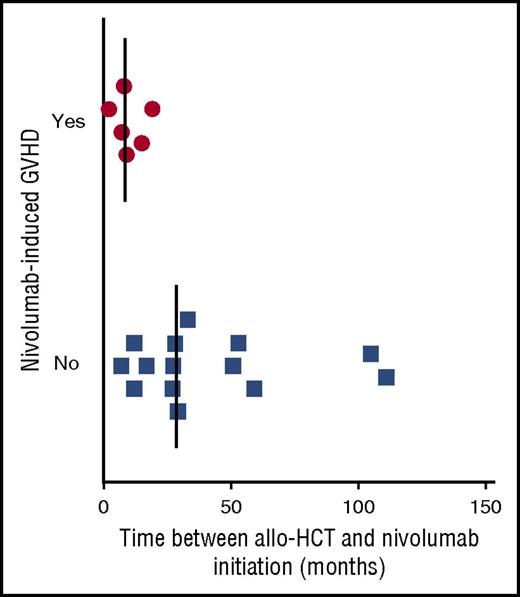
Nivolumab-induced GVHD occurred in 6 patients (30%; 95% CI, 9.9%-50.1%), prompting discontinuation after a single infusion. GVHD localization, grade, time of onset from nivolumab initiation, management, and donor types are listed in Table 2. All cases of aGVHD occurred within 1 week after the first infusion of nivolumab. All 6 patients who presented with aGVHD after nivolumab had a prior history of aGVHD. We did not observe GVHD among patients without history of GVHD. In addition, nivolumab did not trigger a flare of cGVHD in our cohort. As shown in Figure 1, time between allo-HCT and nivolumab treatment was significantly shorter in patients who presented with nivolumab-induced GVHD (median, 8.5 months [range, 2-19 months] vs median, 28.5 months [range, 7-111 months]; P = .0082). No correlation was found between GVHD localization or grade and time of GVHD onset after nivolumab (data not shown). Overall, nivolumab-induced GVHD was successfully managed by using standard treatment for aGVHD.
Precocity of nivolumab initiation seems to have an impact on the development of nivolumab-induced GVHD. Relationship between the presence of a nivolumab-induced GVHD and the time from allo-HCT to nivolumab initiation.
Precocity of nivolumab initiation seems to have an impact on the development of nivolumab-induced GVHD. Relationship between the presence of a nivolumab-induced GVHD and the time from allo-HCT to nivolumab initiation.
Among the 6 patients who developed a nivolumab-induced GVHD, 1 patient (patient 5) quickly developed a febrile multiple organ dysfunction syndrome. No clear etiology has been found and no immunosuppressive treatment was started. This patient died 3 weeks after nivolumab infusion, probably of nivolumab-induced GVHD, even though a classic aGVHD cannot be ruled out. Two patients (patients 2 and 4) were successfully managed by 2 mg/kg corticosteroids. Three patients (patients 1, 3, and 6) presented with steroid-refractory nivolumab-induced GVHD. In patient 1, this was eventually controlled with the addition of intravenous cyclosporine (grade III liver GVHD). Patient 6 received one injection of basiliximab followed by extracorporeal photopheresis (grade IV skin GVHD). Patient 3 refused extracorporeal photopheresis and died of liver GVHD.
Interestingly, immunosuppressive treatment of nivolumab-induced GVHD did not blunt the efficacy of nivolumab on HL; indeed, 3 of 6 patients with nivolumab-induced GVHD are still in response after only 1 injection of nivolumab. In addition, nivolumab did not modify chimerism after allo-HCT (data not shown).
Overall safety profile
The only serious hematologic adverse events were grade 4 neutropenia (n = 1) and grade 3 thrombocytopenia (n = 1, same patient). No nonhematologic adverse events were observed except a grade 2 cerebellar ataxia, which spontaneously resolved 4 days after nivolumab interruption. Four deaths occurred: 2 patients died as a result of GVHD (patients 3 and 5), 1 of progressive disease (patient 6), and one of the complications related to a second allo-HCT performed after relapse (patient 15). Causes of permanent nivolumab discontinuation were as follows: second allo-HCT (n = 1), lack of response (n = 1), hematologic toxicity (after five nivolumab infusions; n = 1), cerebellar ataxia (n = 1), disease progression (n = 3), or nivolumab-induced GVHD (n = 6). Patient 7 transiently discontinued nivolumab because of possibly related grade 2 hepatic cytolysis. The patient relapsed 2 months later, and nivolumab was then restarted leading to a second CR without toxicity at his latest follow-up visit. At the time of this report, 7 patients (35%) remain on nivolumab.
Response to nivolumab after allo-HCT and outcome
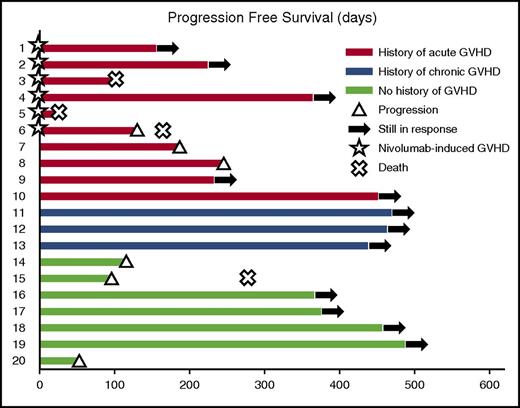
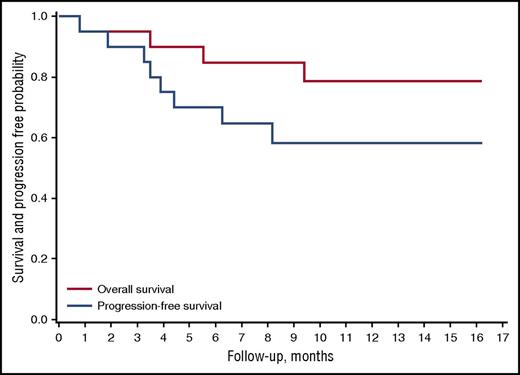
Nineteen of 20 patients were evaluable for response (patient 5 could not be evaluated). Best overall response rate was 95% (95% CI, 74%-100%; n = 18). The CR and PR rates were 42% (95% CI, 21%-67%; n = 8) and 52% (95% CI, 29%-76%; n = 10), respectively. Five relapses occurred after a median time of 132 days (range, 98-245 days). At a median follow-up of 370 days (range, 24-486 days), median PFS and OS had not been reached. The probability of PFS at 12 months was 58.2% (95% CI, 33.1%-76.7%) (Figures 2 and 3). The probability of OS at 12 months was 78.7% (95% CI, 52.4%-91.5% (Figure 3).
Response characteristics in patients with HL receiving nivolumab for a relapse after allo-HCT. PFS of the 20 patients included in this study. The color of each bar indicates the presence and the type of GVHD history. The length of the bar shows the time between nivolumab initiation and progression or death (regardless of the cause).
Response characteristics in patients with HL receiving nivolumab for a relapse after allo-HCT. PFS of the 20 patients included in this study. The color of each bar indicates the presence and the type of GVHD history. The length of the bar shows the time between nivolumab initiation and progression or death (regardless of the cause).
Survival and duration of response. Overall survival and progression-free survival after nivolumab initiation.
Survival and duration of response. Overall survival and progression-free survival after nivolumab initiation.
Discussion
Here we present the outcome of the largest cohort of HL patients treated with a PD-1 inhibitor after allo-HCT including 10 patients with a history of aGVHD. We confirmed the efficacy of nivolumab in the setting of HL relapsing after allo-HCT, with an overall response rate of 95% and a median PFS not reached at a median follow-up of 370 days. At this point, PFS reached a plateau with 12 patients in first ongoing response, and a thirteenth patient in durable response after re-treatment with nivolumab. Among these 13 patients, six remain disease-free despite discontinuation of nivolumab. This substantial clinical activity occurred in a very heavily pretreated patient whose median time to disease progression after allo-HCT was short (8.5 months; Table 1) and required initiation of another treatment before nivolumab in 65% of patients. Of note, nivolumab was effective despite secondary immunosuppression (previous chemotherapy, conditioning regimen, GVHD prevention, and sometimes ongoing corticosteroid therapy). Toxicity of nivolumab after allo-HCT was manageable with careful monitoring and close clinical assessment.
Current strategies for relapsed HL after allo-HCT (summarized in Table 3) are discouraging.19-25 Using different approaches (donor lymphocyte infusion with or without chemotherapy, brentuximab vedotin, or bendamustine), modest results were reported, with median PFS ranging from 6 to 18 months. Although our study did not directly compare patients treated with alternative approaches, our results would suggest at least a better risk-benefit ratio than current strategies for relapsed HL after allo-HCT: median PFS was not reached at 16 months alongside an acceptable rate of 30% GVHD and 9 (45%) long-term responders free of cGVHD whereas a majority of long-term responders developed extensive cGVHD after donor lymphocyte infusion–based approaches.
Tempering this encouraging result, nivolumab can potentially induce severe, steroid-refractory GVHD in keeping with the data of the 2 known murine models.8,9 In this study, nivolumab-induced GVHD occurred in 6 patients (30%). Two patients died of this GVHD. It was controlled with corticosteroids in 2 patients, whereas it was steroid-refractory and finally responded to alternative immunosuppressive therapies in 2 patients. All 6 patients had previously developed aGVHD, which might reflect greater alloreactivity. Conversely, nivolumab did not induce GVHD in patients who lacked a prior history of aGVHD. In light of these observations, we advise physicians to carefully weigh the benefits against the risks in patients with a prior history of aGVHD. Follow-up visits after administration of nivolumab should be carried out by physicians with expertise in allo-HCT and management of GVHD.
In patients without a history of aGVHD, our results are consistent with those of the 3 case reports published so far12-14 that altogether described 4 patients treated with PD-1 blockade therapy for HL after allo-HCT, with 4 responses to nivolumab and no GVHD after treatment.
Intriguingly, nivolumab did not induce cGVHD in any patient in our cohort. Moreover, it did not induce a flare in the 4 patients with controlled cGVHD. These findings conflict with the murine data from Fujiwara et al,26 who showed that blockade of the PD-1 pathway exacerbated cGVHD. These discrepancies highlight different pathophysiology between human and murine cGVHD. One hypothesis could be that the PD-1/programmed death-ligand 1 (PDL-1) pathway has no or little role in cGVHD in humans.
On the basis of these results, we believe that nivolumab is a suitable option for treating HL relapsing after allo-HCT, with a potentially better risk-benefit ratio compared with other therapeutic options, such as brentuximab vedotin and donor lymphocyte infusion. Nonetheless, nivolumab therapy requires careful monitoring and close clinical assessment of GVHD by the bone marrow transplant team, particularly if the patient needs to be treated early, especially within 1 year after allo-HCT, and if the patient has a prior history of aGVHD. For all patients, we recommend a systematic clinical and biological evaluation 1 week after nivolumab initiation. If GVHD is suspected, we recommend, along with diagnostic procedures, immediate treatment with systemic corticosteroids at 2 mg/kg. If GVHD does not improve within 24 to 48 hours, alternative immunosuppressive therapies must be quickly initiated. We acknowledge the limitations of our study, given its retrospective nature and the small number of patients studied. Our encouraging results should be confirmed prospectively in a larger cohort. Furthermore, the acceptable safety profile described here should pave the way to investigate treatment with nivolumab after allo-HCT in other hematologic malignancies.
Presented in part at the 57th annual meeting of the American Society of Hematology, Orlando, FL, December 5-8, 2015.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank their families and their patients.
Authorship
Contribution: C.H., J.G., I.Y.-A., and F.M. contributed to the overall design, performed research, collected, analyzed, and interpreted data, and prepared and wrote the manuscript; E.D., C.H., and F.M. analyzed the data and performed statistical analyses; C.H., P.B., L.Y., H. Doyen, L.F., K.B., G.M., H.G., R.T., E.H., J.L., A.T.-B., A.C., and F.M. collected data; E.B., H. Demarquette, and R.H. provided support; C.H. and F.M. provided final review of the manuscript; and all authors critically reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Franck Morschhauser, Lille University Hospital, Hematology Department, Rue Michel Polonovski, F-59037 Lille CEDEX, France; e-mail: franck.morschhauser@chru-lille.fr.