Key Points
Antibiotics cause multilineage alterations in murine hematopoiesis, with marked suppression of multipotent progenitors.
Suppression of bone marrow populations results from depletion of intestinal microbiota.
Abstract
Bone marrow suppression is an adverse effect associated with many antibiotics, especially when administered for prolonged treatment courses. Recent advances in our understanding of steady-state hematopoiesis have allowed us to explore the effects of antibiotics on hematopoietic progenitors in detail using a murine model. Antibiotic-treated mice exhibited anemia, thrombocytosis, and leukopenia, with pronounced pan-lymphopenia as demonstrated by flow cytometric analysis of peripheral blood. Bone marrow progenitor analysis revealed depletion of hematopoietic stem cells and multipotent progenitors across all subtypes. Granulocytes and B cells were also diminished in the bone marrow, whereas the number of CD8+ T cells increased. Reductions in progenitor activity were not observed when cells were directly incubated with antibiotics, suggesting that these effects are indirect. Hematopoietic changes were associated with a significant contraction of the fecal microbiome and were partially rescued by fecal microbiota transfer. Further, mice raised in germ-free conditions had hematopoietic abnormalities similar to those seen in antibiotic-treated mice, and antibiotic therapy of germ-free mice caused no additional abnormalities. The effects of antibiotics were phenocopied in Stat1-deficient mice, with no additional suppression by antibiotics in these mice. We conclude that microbiome depletion as a result of broad-spectrum antibiotic treatment disrupts basal Stat1 signaling and alters T-cell homeostasis, leading to impaired progenitor maintenance and granulocyte maturation. Methods to preserve the microbiome may reduce the incidence of antibiotic-associated bone marrow suppression.
Introduction
Approximately 270 million antibiotic prescriptions are issued in the United States yearly.1 Treatment courses >2 weeks in duration are used for a number of conditions including endocarditis and osteomyelitis, and bone marrow suppression, manifesting as anemia, thrombocytopenia, and/or neutropenia, is a major adverse effect of prolonged administration of antibiotics. β-lactam antibiotics such as penicillin are most commonly implicated in these occurrences. Neutropenia has been reported in as much as 15% of patients receiving high-dose β-lactam antibiotics for >2 weeks,2 typically in the third or fourth week of treatment.3 When bone marrow suppression goes unrecognized, it may result in additional complications such as superinfection,4 but it usually improves rapidly when the antibiotic is discontinued.3 Therefore, regular blood testing is required to monitor for these complications, significantly increasing the total cost of therapy. Despite its broad impact, the exact mechanism of antibiotic-associated bone marrow suppression has never been established. A better understanding of this process may identify preventive measures and more focused monitoring for myelosuppression, resulting in improved patient care and decreased health care expenditures.
In one of the few studies to investigate the pathophysiology of antibiotic-induced bone marrow suppression, profound neutropenia attributed to antibiotic therapy was found to be associated with severe hypocellularity of the marrow.5 These results support a suppressive effect by antibiotics on hematopoietic progenitor function. However, previous studies have not identified the specific progenitor cell populations and mechanisms by which they are affected.
New knowledge in the field of hematopoiesis may provide insight into the etiology of antibiotic-associated bone marrow suppression. For example, recent publications have highlighted the pivotal role multipotent progenitors (MPPs) play in steady-state hematopoiesis,6-9 but the effects of antibiotics on these subpopulations have not been defined. Also, a variety of systemic and microenvironmental signals have been shown to affect proliferation, differentiation, and activity, as well as retention of hematopoietic stem and progenitor cells (HSPCs) in the marrow.10-12 Recent studies have even demonstrated effects of the intestinal microbiome on hematopoiesis.13,14 For instance, the intestinal microbiome, suppressible with antibiotic therapy, plays an important role in stimulating bone marrow production of granulocytes15 as well as influencing activation and possibly retention of granulocytes in the circulation.16 Changes in inflammatory milieu or commensal microbiota with antibiotic therapy could therefore have significant extrinsic influence on hematopoiesis.
In this study, we aimed to develop an improved understanding of which hematopoietic progenitor subsets are most affected by antibiotic therapy. By defining the mechanisms underlying antibiotic-associated cytopenia, we hope to discover methods to prevent this complication.
Methods
Mice
Six- to 15-week-old CD45.1 or CD45.2 C57BL/6 mice were used for all experiments. Mice were bred and housed in specific pathogen-free (SPF) conditions at animal facilities at Baylor College of Medicine (BCM) or Washington University in accordance with protocols approved by the respective Institutional Animal Care and Use Committees. B6.129P2(SJL)-Myd88tm1.1Defr/J stock #009088 (MyD88 knockout) mice, B6.129S(Cg)-Stat1tm1Div/J stock #012606 (Stat1 knockout) mice, and B6.129S1-Nod2tm1Flv/J Stock #005763 (Nod2 knockout) mice were obtained from The Jackson Laboratory, maintained in breeding colonies, and treated at Washington University. Germ-free C57BL/6J mice were obtained from the Germ-Free Animal Facility at the BCM Center for Comparative Medicine and transferred to SPF housing for antibiotic therapy.
Antibiotic therapy
Mice were fed water ad libitum containing antibiotics: 1 g/L ampicillin, 0.5 g/L vancomycin, 1 g/L neomycin, and 1 g/L metronidazole, all obtained from Sigma. Ciprofloxacin 1g/L (Sigma) was used in some experiments. Flavoring (20 g/L grape-flavored Kool-Aid Drink Mix [Kraft Foods Global, Inc.]) was added to the drinking water for all groups. Treatment was continued for 14 days.
Complete blood counts
Mice were bled at 14 days of therapy and analyzed using an Advia 120 automated hematology system (Siemens).
Flow cytometry for blood and bone marrow populations
Bone marrow was obtained by flushing leg bones. Blood and bone marrow cells were stained (see supplemental Methods for details, available on the Blood Web site) and analyzed on a LSRII flow cytometer (BD Biosciences).
Proliferation, cell cycle, and apoptosis flow cytometry assays
Proliferation of cell populations in bone marrow was assessed using the BD Pharmingen FITC BrdU flow kit (BD-Pharmingen) according to the package insert. Cell cycle activity was assessed using cell fixing and permeabilization reagents from the BD Pharmingen BrdU flow kit (BD-Pharmingen) and anti–Ki-67 antibody conjugated to fluorescein isothiocyanate (FITC) (clone SolA15, eBioscience). Apoptosis was assessed with fluorescein isothiocyanate–conjugated annexin V staining (BD Biosciences) of pooled marrow after lineage depletion using biotin-conjugated antibodies against lineage markers (CD3, CD4, CD8, CD11b, Ly6G, Ter119, B220), anti–biotin-magnetic beads (Miltenyi BioTech), and AutoMACS (Miltenyi BioTech).
Methylocellulose and OP9-DL1 culture
Murine whole bone marrow (WBM) was cultured in methylcellulose with appropriate murine cytokines (MethoCult M3434, Stemcell Technologies). Three-thousand three hundred WBM cells per well were plated in a 12-well tissue culture plate and incubated at 37°C, 5% CO2 for 9 days before colonies were counted.
OP9-DL1 coculture was conducted according to the method of Holmes et al.17 Lineage–Sca1+cKit+ HSPCs were incubated on an OP9-DL1 monolayer in the presence of 5 ng/mL Flt3L and 10 ng/mL IL7 with half media changes every 4 days and evaluated by flow cytometry at day 14.
G-CSF treatment
Control or antibiotic-treated mice were treated with 250 mg/kg granulocyte-colony stimulating factor (G-CSF) (Amgen) by subcutaneous injection daily for 3 days on days 11 to 13 of antibiotic treatment before analysis on days 14 to 15.
Stool microbiome evaluation
Fecal pellets were collected from individual mice and stored at −80°C. SYBR green quantitative polymerase chain reaction for 16S was performed with 515F (5′- GTGCCAGCMGCCGCGGTAA-3′) and 805R (5′-GACTACCAGGGTATCTAATCC-3′) primers on phenol:chloroform–extracted DNA from mouse fecal pellets, as previously described.18
Fecal microbiota transfer
Antibiotic-treated and control mice were transferred to separate, clean, empty cages daily for fecal pellet collection. Three pellets per cage were suspended in 1.5 mL phosphate-buffered saline. One-hundred μL fecal suspension was immediately pipetted into the oropharynx of the recipients.
Statistical analysis
Results were analyzed using a 2-tailed Student t test comparing means for untreated and antibiotic-treated groups, or Mann-Whitney test for skewed data. Results with P < .05 were considered statistically significant.
Full methods are available in the supplemental Materials.
Results
Antibiotic treatment suppresses peripheral blood counts
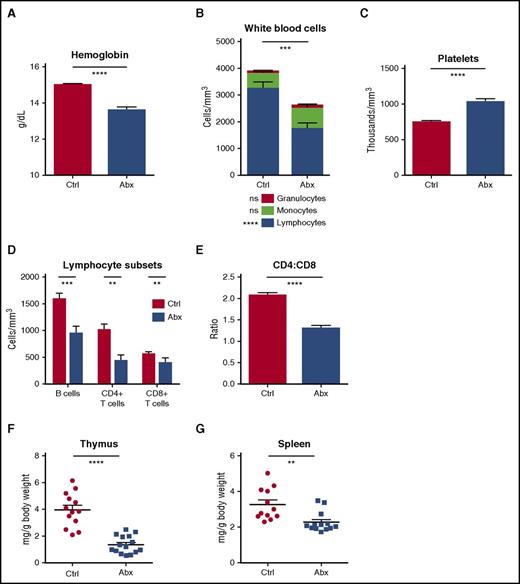
To evaluate the effects of antibiotics on peripheral blood, we treated mice with a 4-antibiotic cocktail of vancomycin, neomycin, ampicillin, and metronidazole (VNAM) in the drinking water for 2 weeks. Compared with control mice, antibiotic-treated mice became anemic and leukopenic (Figure 1A-B; supplemental Figure 1A), and platelet counts were significantly elevated (Figure 1C). There were no changes in the absolute number of peripheral granulocytes with antibiotic therapy. However, antibiotic-treated mice developed significant lymphopenia. B cells and CD4+ and CD8+ T cells were all diminished, and CD4+ T cells were especially low, manifest as a decreased CD4:CD8 ratio (Figure 1D-E; supplemental Figure 1B). Consistent with these findings, peripheral lymphoid tissues involuted with antibiotic therapy (Figure 1F-G).
Peripheral blood counts are suppressed and lymphoid organs diminished with antibiotic therapy. (A) Hemoglobin, (B) white blood cell count and differential, and (C) platelet counts were determined for control (Ctrl) vs antibiotic-treated (Abx) mice using an automated hematologic cell counter. (D) Lymphocyte subsets and (E) CD4:CD8 ratio in peripheral blood for control vs antibiotic-treated mice were determined by flow cytometry. (F) Thymi and (G) spleens from control vs antibiotic-treated mice were weighed. Results are compiled from 3 independent experiments (n = 9-14 per group). Graphs show mean + standard error of the mean (SEM). Statistical significance was determined by Student t test. ns, not significant; *P < .05, **P < .01, ***P < .001, ****P < .0001.
Peripheral blood counts are suppressed and lymphoid organs diminished with antibiotic therapy. (A) Hemoglobin, (B) white blood cell count and differential, and (C) platelet counts were determined for control (Ctrl) vs antibiotic-treated (Abx) mice using an automated hematologic cell counter. (D) Lymphocyte subsets and (E) CD4:CD8 ratio in peripheral blood for control vs antibiotic-treated mice were determined by flow cytometry. (F) Thymi and (G) spleens from control vs antibiotic-treated mice were weighed. Results are compiled from 3 independent experiments (n = 9-14 per group). Graphs show mean + standard error of the mean (SEM). Statistical significance was determined by Student t test. ns, not significant; *P < .05, **P < .01, ***P < .001, ****P < .0001.
Bone marrow cells are depleted in antibiotic-treated mice
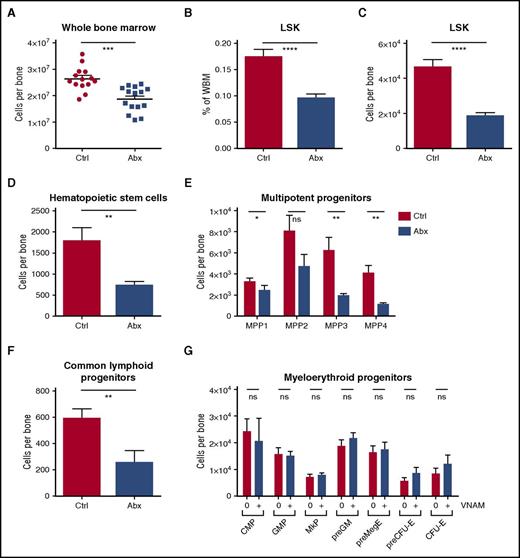
To evaluate effects of antibiotics on hematopoiesis, we examined the bone marrow by flow cytometry. WBM counts were decreased in the antibiotic-treated mice (Figure 2A). Hematopoietic progenitors (lineage– Sca1+ cKit+ [LSK]) were significantly decreased both as a percentage of WBM and in absolute numbers (Figure 2B-C). Among these, all primitive hematopoietic progenitors, including hematopoietic stem cells (LSK CD150+ CD48– CD34– Flk2–) and multipotent progenitors (LSK CD48+/− CD34+ CD150+/− Flk2+/−) were significantly depleted (Figure 2D-E). The common lymphoid progenitors (lineage– IL7Ra+ cKitlo Flk2+ Sca1+) were also severely reduced with antibiotic therapy (Figure 2F), consistent with the decrease in peripheral lymphocytes. Despite the loss of MPPs, myeloid progenitors (MPs) including common myeloid progenitors (lineage– IL7Ra– cKit+ Sca1– CD34+ CD16/32–), granulocyte-monocyte precursors (lineage– cKit+ Sca1– CD41– CD16/32+), megakaryocyte progenitors (lineage– cKit+ Sca1– CD150+ CD41+), premegakaryocyte/erythrocytes (lineage– cKit+ Sca1– CD150+ CD16/32– CD105-), and pre-erythroid colony-forming units (lineage– cKit+ Sca1– CD150+ CD16/32– CD105+) were conserved (Figure 2G). In fact there was a small but statistically significant increase in myeloid colony formation in methylcellulose with antibiotic treatment, consistent with an increase in the percentage of MPs in the setting of loss of other cell types from the bone marrow (supplemental Figure 2A). Representative flow plots for all progenitor populations are shown in supplemental Figure 2B-D.
WBM counts are suppressed and lineage balance is shifted with antibiotic therapy. (A) Bone marrow cells were counted on a hemocytometer in control (Ctrl) vs antibiotic-treated (Abx) mice. (B-G) Progenitor populations were quantified by flow cytometry in controls vs antibiotic-treated mice. (B) The percentage of total primitive progenitors (LSK) was determined by flow cytometry. Absolute numbers of (C) LSK progenitors, (D) hematopoietic stem cells, and (E) multipotent progenitor subsets, (F) common lymphoid progenitors, and (G) committed myeloid progenitor subsets are shown. Results are compiled from 4 independent experiments (n = 9-17 per group). Graphs show mean + SEM. Statistical significance was determined by Student t test or Mann-Whitney U test. *P < .05, **P < .01, ***P < .001, ****P < .0001.
WBM counts are suppressed and lineage balance is shifted with antibiotic therapy. (A) Bone marrow cells were counted on a hemocytometer in control (Ctrl) vs antibiotic-treated (Abx) mice. (B-G) Progenitor populations were quantified by flow cytometry in controls vs antibiotic-treated mice. (B) The percentage of total primitive progenitors (LSK) was determined by flow cytometry. Absolute numbers of (C) LSK progenitors, (D) hematopoietic stem cells, and (E) multipotent progenitor subsets, (F) common lymphoid progenitors, and (G) committed myeloid progenitor subsets are shown. Results are compiled from 4 independent experiments (n = 9-17 per group). Graphs show mean + SEM. Statistical significance was determined by Student t test or Mann-Whitney U test. *P < .05, **P < .01, ***P < .001, ****P < .0001.
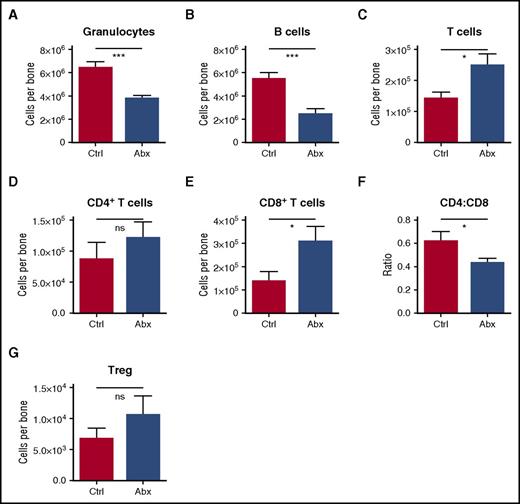
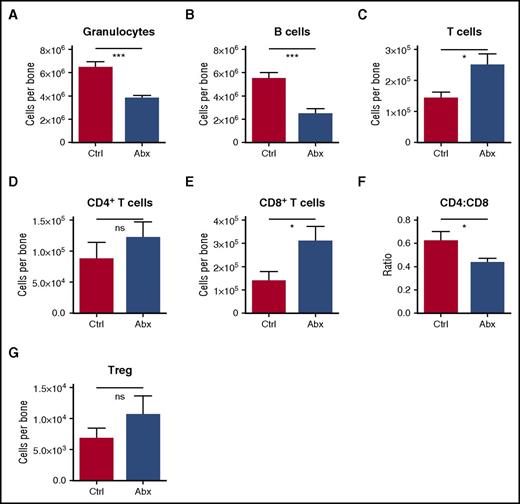
Among differentiated leukocytes in the bone marrow, granulocytes and B cells were decreased (Figure 3A-B). However, T cells were increased in the bone marrow, primarily accounted for by an increase in CD8+ T cells, with a resulting decrease in CD4:CD8 ratio (Figure 3C-F). Most of the CD8 T cells in the marrow were naïve (CD44lo, CD62Lhi) (supplemental Figures 3A-C). Meanwhile, there was no significant change in the number of T-regulatory cells in the bone marrow (Figure 3G). Altogether, these findings indicate that antibiotic treatment causes significant alterations in the hematopoietic system, with decreased bone marrow cellularity accounted for by a loss of granulocytes, B cells, and all primitive progenitors, but sparing the MPs.
Granulocytes and B cells decrease in bone marrow upon antibiotic therapy, whereas T cells increase. Mature blood cells in bone marrow of control (Ctrl) vs antibiotic-treated (Abx) mice were quantified by flow cytometry, including (A) granulocytes, (B) B cells, (C) T cells, (D) CD4+ T cells, and (E) CD8+ T cells. (F) The CD4:CD8 ratio was calculated based on percentages of each population. (G) Regulatory T cells (Treg) were quantified by flow cytometry. Results are compiled from 2 to 4 independent experiments (n = 9-17 per group). Graphs show mean + SEM, with statistical significance determined by Student t test. *P < .05, ***P < .001.
Granulocytes and B cells decrease in bone marrow upon antibiotic therapy, whereas T cells increase. Mature blood cells in bone marrow of control (Ctrl) vs antibiotic-treated (Abx) mice were quantified by flow cytometry, including (A) granulocytes, (B) B cells, (C) T cells, (D) CD4+ T cells, and (E) CD8+ T cells. (F) The CD4:CD8 ratio was calculated based on percentages of each population. (G) Regulatory T cells (Treg) were quantified by flow cytometry. Results are compiled from 2 to 4 independent experiments (n = 9-17 per group). Graphs show mean + SEM, with statistical significance determined by Student t test. *P < .05, ***P < .001.
To test the functional capacity of the remaining bone marrow progenitors, we conducted WBM transplant assays to compare engraftment of treated and untreated progenitors in naïve recipient animals. The marrow of antibiotic-treated mice did not show any deficits in engraftment over a 16-week period post-transplant, and the lineage distribution of engrafted cells was also similar. These studies demonstrate that although the bone marrow cellularity was reduced during antibiotic treatment, the functional capacity of the remaining progenitors was conserved (supplemental Figure 4A-F).
Antibiotic therapy does not trigger apoptosis of bone marrow progenitors
We next sought to investigate the mechanisms of progenitor loss during antibiotic treatment. To evaluate whether antibiotics deplete hematopoietic progenitors by triggering apoptosis, we measured annexin V staining in progenitor populations isolated from antibiotic-treated mice. There was no significant difference in apoptosis of primitive, myeloid or lymphoid progenitor populations in the bone marrow of treated mice compared with controls (supplemental Figure 5A-C).
Antibiotic treatment suppresses cell cycle activity in progenitor populations
To determine whether antibiotics inhibit proliferation of hematopoietic cells, we assessed incorporation of BrdU label in progenitor populations. Among hematopoietic stem cells (HSCs) and lineage– cKit+ Sca1– (LK) cells, a population comprised primarily of MPs, BrdU incorporation was increased in antibiotic-treated mice compared with controls as expected in the setting of low bone marrow cellularity19 (Figure 4A-B). However, BrdU incorporation in total progenitor (LSK) and MPP populations was not altered by antibiotic therapy (Figure 4C-D). Ki67 staining trended upward in MPs (lineage– IL7Ra– cKit+ Sca1–; supplemental Figure 6) but was decreased among LSK progenitors, which include the MPPs (Figure 4E). Collectively, these results indicate that the rate of cell division was increased among MPs and HSCs but not the MPPs, a class of progenitors particularly important for steady state hematopoiesis.6,7 The lack of increased cell division among MPPs despite significantly decreased numbers of MPPs and common lymphoid progenitor (CLPs) indicates a major loss of homeostasis among the primitive hematopoietic progenitors during antibiotic treatment.
Antibiotics suppress cell cycle activity in primitive progenitor populations. (A-B) BrdU incorporation was determined for controls (Ctrl) vs antibiotic-treated (Abx) mice in (A) stem cells, (B) MPs, (C) LSK, and (D) primitive progenitor subsets in bone marrow. Results are compiled from 2 independent experiments (n = 9 per group per analysis). (E) Ki67 staining was determined by flow cytometry in the total LSK population. Results are compiled from 3 independent experiments, each performed on pooled marrow from 4 to 5 mice per group. Graphs show mean + SEM. Statistical significance was determined by Student t test. *P < .05, **P < .01.
Antibiotics suppress cell cycle activity in primitive progenitor populations. (A-B) BrdU incorporation was determined for controls (Ctrl) vs antibiotic-treated (Abx) mice in (A) stem cells, (B) MPs, (C) LSK, and (D) primitive progenitor subsets in bone marrow. Results are compiled from 2 independent experiments (n = 9 per group per analysis). (E) Ki67 staining was determined by flow cytometry in the total LSK population. Results are compiled from 3 independent experiments, each performed on pooled marrow from 4 to 5 mice per group. Graphs show mean + SEM. Statistical significance was determined by Student t test. *P < .05, **P < .01.
Antibiotic therapy does not have direct suppressive effects on hematopoietic progenitors
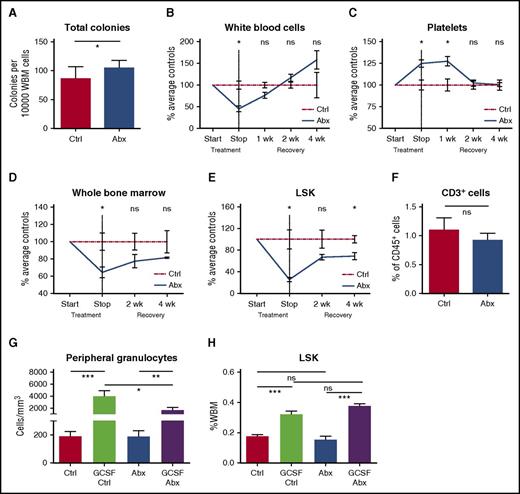
To determine whether antibiotic compounds directly suppress progenitor activity, we cultured murine bone marrow in methylcellulose with and without antibiotics, and assessed colony-forming ability. When untreated bone marrow was exposed to the β-lactam antibiotic ceftriaxone in methylcellulose culture, there was no significant effect on colony formation (supplemental Figure 7A). Bone marrow was also cultured with the antibiotic cocktail used for in vivo experiments at concentrations exceeding those expected in vivo. These studies showed that colony formation was stable or increased in the direct presence of antibiotics, confirming that the antibiotics did not exert direct toxic effects on progenitor growth and survival (Figure 5A).
Antibiotic suppression of bone marrow progenitors is indirect and reversible. (A) Total colony formation was measured for WBM cells incubated for 9 days in methylcellulose in the presence (Abx) or absence (Ctrl) of test antibiotics (VNAM). Results compiled from 2 independent experiments. Graph shows mean + SEM. (B-E) Recovery of cell populations after withdrawal of antibiotics. Peripheral blood cell count recovery was assessed on an automated hematologic cell counter for antibiotic-treated (Abx) mice compared with controls (Ctrl), exemplified by (B) white blood cells and (C) platelets. Bone marrow recovery was assessed by performing (D) whole bone marrow counts and flow cytometry quantification of bone marrow populations, exemplified by (E) total LSK population, in antibiotic-treated mice compared with controls. Graphs show mean ± SEM trend over time (n = 3-4 per group per time point with each time point normalized to the mean of controls for that time point). Single experiment. (F) Sorted LSK cells from controls (Ctrl) and antibiotic-treated (Abx) mice were cocultured with OP9-DL1 cells and cytokines to support T-cell development. Flow cytometry was performed after 14 days in culture to quantify CD3+ cells representing lymphoid potential of the sorted LSK cells. The graph shows mean + SEM. (G-H) Controls (Ctrl) and antibiotic-treated (Abx) mice were treated with granulocyte-colony stimulating factor (G-CSF) to assess progenitor response to exogenous stimuli of myeloid differentiation. (G) Peripheral blood granulocyte and (H) total BM LSK response to GCSF. Results are compiled from 2 independent experiments (n = 4-8 per group per analysis). The graphs show mean + SEM. For all panels, statistical significance was determined by Student t test. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Antibiotic suppression of bone marrow progenitors is indirect and reversible. (A) Total colony formation was measured for WBM cells incubated for 9 days in methylcellulose in the presence (Abx) or absence (Ctrl) of test antibiotics (VNAM). Results compiled from 2 independent experiments. Graph shows mean + SEM. (B-E) Recovery of cell populations after withdrawal of antibiotics. Peripheral blood cell count recovery was assessed on an automated hematologic cell counter for antibiotic-treated (Abx) mice compared with controls (Ctrl), exemplified by (B) white blood cells and (C) platelets. Bone marrow recovery was assessed by performing (D) whole bone marrow counts and flow cytometry quantification of bone marrow populations, exemplified by (E) total LSK population, in antibiotic-treated mice compared with controls. Graphs show mean ± SEM trend over time (n = 3-4 per group per time point with each time point normalized to the mean of controls for that time point). Single experiment. (F) Sorted LSK cells from controls (Ctrl) and antibiotic-treated (Abx) mice were cocultured with OP9-DL1 cells and cytokines to support T-cell development. Flow cytometry was performed after 14 days in culture to quantify CD3+ cells representing lymphoid potential of the sorted LSK cells. The graph shows mean + SEM. (G-H) Controls (Ctrl) and antibiotic-treated (Abx) mice were treated with granulocyte-colony stimulating factor (G-CSF) to assess progenitor response to exogenous stimuli of myeloid differentiation. (G) Peripheral blood granulocyte and (H) total BM LSK response to GCSF. Results are compiled from 2 independent experiments (n = 4-8 per group per analysis). The graphs show mean + SEM. For all panels, statistical significance was determined by Student t test. *P < .05, **P < .01, ***P < .001, ****P < .0001.
We further tested whether the suppressive effects of antibiotics were dependent on the type of antibiotics administered. We treated mice with the standard cocktail of VNAM or two alternative antibiotic regimens: vancomycin, neomycin, and metronidazole (VNM) or ciprofloxacin and metronidazole (ciproM), which are similar to antibiotic combinations used in patients. Effects of antibiotic treatment on the bone marrow were equivalent regardless of the antibiotic regimen used; in all cases, the WBM count, the progenitor (LSK) count, and the granulocyte count were diminished, whereas the CD8+ T-cell count increased (supplemental Figure 7B-E). Meanwhile, the combination of ampicillin and vancomycin alone, which omits activity against gram-negative anaerobic bacteria, did not have a significant effect on peripheral blood or bone marrow counts (not shown). Collectively, these results indicate that a variety of antibiotic combinations, regardless of their propensity for systemic absorption, yield the observed effects and further support the idea that suppressive effects of antibiotics on hematopoiesis are not attributable to direct toxic effects by antibiotics or their metabolic derivatives.
Detrimental effects of antibiotic therapy on hematopoiesis are reversible
To assess whether antibiotics cause permanent bone marrow damage, we observed mice for up to 4 weeks after cessation of antibiotic treatment. Changes in the peripheral blood, including leukopenia, increased platelets, and a lower CD4:CD8 ratio, recovered within 2 to 4 weeks after antibiotics were stopped (Figure 5B-C). The total bone marrow count improved, and bone marrow progenitors (LSK) showed a trend toward recovery at 4 weeks after discontinuation of the antibiotics (Figures 5D-E).
We next tested whether lymphoid differentiation capacity could be rescued under in vitro growth conditions. We conducted coculture assays of progenitors (LSK) from control and antibiotic-treated animals with OP9-DL1 cells in the presence of Flt3 ligand and IL7. The number of CD3+ T cells derived from the sorted progenitors was equivalent between the control and antibiotic-treated groups (Figure 5F), indicating that environmental factors, rather than clonogenic potential, were suppressed by antibiotic treatment.
We further tested whether emergency granulopoiesis was impaired in the antibiotic-treated animals. We treated control and antibiotic-treated mice with G-CSF. The response to G-CSF was intact, with a significant increase of bone marrow progenitors (LSK) and peripheral blood granulocytes in both control and antibiotic-treated mice (Figure 5G-H). Together with the WBM transplant assays described before, these results demonstrate that progenitor populations were not permanently damaged by antibiotic treatment. Instead, our results suggest that antibiotic treatment changes the cytokine milieu required for homeostasis of the hematopoietic system, and that suppressed hematopoietic function can be overcome by recovery of these environmental factors or exogenous addition of appropriate stimulatory cytokines.
Antibiotic therapy depletes fecal microbiota
Considering that the effects of antibiotic therapy appeared to be indirect, we investigated the effects of antibiotics on the microbiome and the possible involvement of the microbiome in hematopoietic changes. We evaluated the fecal microbiome by 16S rDNA quantitative polymerase chain reaction. As expected, the abundance of fecal microbes was dramatically decreased after antibiotic therapy (supplemental Figure 8A). Given that the fecal microbiota may influence the basal inflammatory state of host tissues, particularly in the intestines,20,21 we wondered whether antibiotic therapy altered basal inflammatory tone in treated animals. We measured systemic levels of common inflammatory cytokines, including several known to influence hematopoiesis such as IFNα, IFNγ, IL6, and tumor necrosis factor, by enzyme-linked immunosorbent assay. Screening the serum by this relatively low sensitivity method did not detect global differences in systemic inflammatory tone (supplemental Figure 8B-F).
Suppression of myelopoiesis by antibiotics is dependent on changes in the microbiome
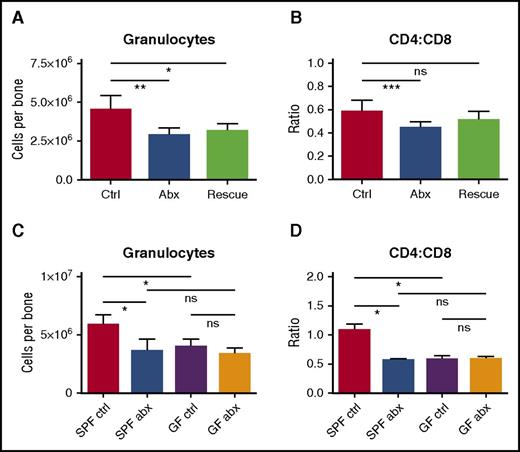
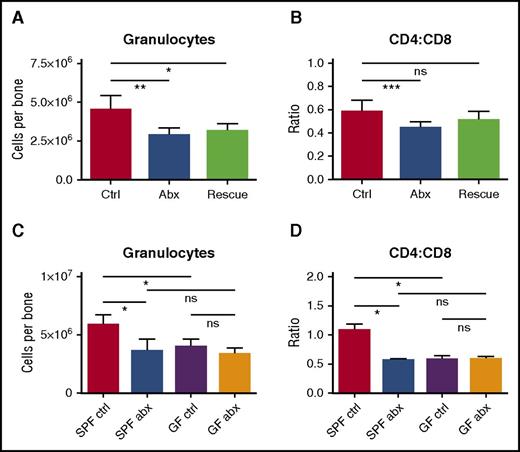
We reasoned that if changes in the microbiome are responsible for altered hematopoiesis, fecal microbiota transfer (FMT) would offset the harmful effects of antibiotics on blood production. We therefore conducted daily FMT “rescue” on mice during antibiotic treatment. Mice fed a suspension of fecal material from control (non–antibiotic-treated) mice had modestly improved bone marrow counts even though they continued to receive antibiotic treatment (Figure 6A-B). These improvements did not reach statistical significance when compared with antibiotic-treated mice without FMT “rescue,” possibly because the microbiome remained suppressed in these mice based on stool culture at the end of therapy (supplemental Figure 8G).
Depletion of fecal microbiota is a key factor in the development of antibiotic-associated bone marrow suppression. (A) Granulocytes and (B) CD4:CD8 ratio were quantified by flow cytometry for controls (Ctrl), antibiotic-treated mice (Abx), and mice treated with antibiotics plus fecal microbiota transfer (Rescue). Results are compiled from 2 independent experiments (n = 5-17 per group). (C) Granulocytes and (D) CD4:CD8 ratio were quantified by flow cytometry for controls and antibiotic-treated SPF and germ-free (GF) mice. Results are representative of a single experiment (n = 3-5 per group per experiment). The graphs show mean + SEM, statistical significance was determined by Student t test. *P < .05, **P < .01, ***P < .001.
Depletion of fecal microbiota is a key factor in the development of antibiotic-associated bone marrow suppression. (A) Granulocytes and (B) CD4:CD8 ratio were quantified by flow cytometry for controls (Ctrl), antibiotic-treated mice (Abx), and mice treated with antibiotics plus fecal microbiota transfer (Rescue). Results are compiled from 2 independent experiments (n = 5-17 per group). (C) Granulocytes and (D) CD4:CD8 ratio were quantified by flow cytometry for controls and antibiotic-treated SPF and germ-free (GF) mice. Results are representative of a single experiment (n = 3-5 per group per experiment). The graphs show mean + SEM, statistical significance was determined by Student t test. *P < .05, **P < .01, ***P < .001.
If antibiotics suppress hematopoiesis through the microbiome, germ-free mice should show the same hematologic defects as antibiotic-treated mice, and treatment with antibiotics should not have further effects on blood or bone marrow counts in germ-free mice. Therefore, we treated germ-free mice with antibiotics for 2 weeks and evaluated blood and bone marrow. As predicted, germ-free mice had decreased granulocytes and increased CD8+ T cells in the bone marrow, similar to the antibiotic-treated SPF mice (Figure 6C-D). Furthermore, antibiotic therapy of the germ-free mice did not further suppress the granulocyte count or increase the CD8+ T-cell count. These findings indicate that hematopoietic changes with antibiotic therapy are mediated through alterations in the microbiome.
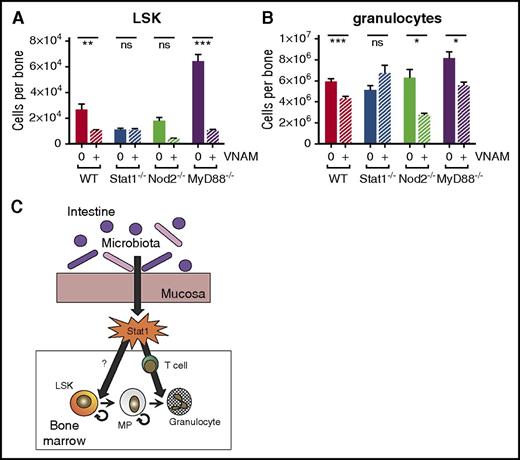
Antibiotic suppression of myelopoiesis depends on Stat1 but not Nod2 or MyD88-dependent Toll-like receptor signaling
To identify signaling pathways that contribute to bone marrow suppression during antibiotic treatment, we evaluated response to antibiotics in knockout (KO) mice with mutations in genes important in microbiome-associated immunomodulation. We treated wild-type (WT), MyD88 KO, Nod2 KO, and Stat1 KO mice with antibiotics for 2 weeks. Hematopoietic defects in antibiotic-treated MyD88 KO and Nod2 KO mice were similar to antibiotic-treated WT mice for both LSK progenitors (Figure 7A) and mature cells (Figure 7B). However, the bone marrow of Stat1-deficient mice phenocopied that of antibiotic-treated mice with low LSK counts, and antibiotics did not suppress granulocyte count in the bone marrow of Stat1-deficient mice (Figure 7A-B). These results suggest that a basal level of Stat1 signaling, stimulated by the microbiome, is required for maintenance of normal bone marrow function.
Stat1 KO mice exhibit no significant population shifts with antibiotic therapy. (A) Total LSK and (B) granulocytes from bone marrow were quantified by flow cytometry for WT, Stat1 KO (Stat1−/−), Nod2 KO (Nod2−/−), and MyD88 KO (MyD88−/−) with (+) and without (0) antibiotic therapy (VNAM). Results are pooled from 2 to 4 independent experiments (n = 4-25 per group per analysis). The graphs show mean + SEM, statistical significance was determined by Student t test. ns, not significant; *P < .05, **P < .01, ***P < .001. (C) Proposed model of antibiotic-associated bone marrow suppression.
Stat1 KO mice exhibit no significant population shifts with antibiotic therapy. (A) Total LSK and (B) granulocytes from bone marrow were quantified by flow cytometry for WT, Stat1 KO (Stat1−/−), Nod2 KO (Nod2−/−), and MyD88 KO (MyD88−/−) with (+) and without (0) antibiotic therapy (VNAM). Results are pooled from 2 to 4 independent experiments (n = 4-25 per group per analysis). The graphs show mean + SEM, statistical significance was determined by Student t test. ns, not significant; *P < .05, **P < .01, ***P < .001. (C) Proposed model of antibiotic-associated bone marrow suppression.
Discussion
In this study, we evaluated the effects of prolonged broad-spectrum antibiotic therapy on hematopoiesis in a murine model. We demonstrated that antibiotic therapy significantly reduced peripheral blood counts and bone marrow cellularity. Antibiotic therapy affected several classes of hematopoietic progenitors, including HSCs, common lymphoid progenitors, and all MPP subtypes. Despite severe suppression of peripheral lymphocytes, naïve CD8+ T cells were increased in bone marrow. These hematopoietic changes were associated with a logarithmic reduction of fecal microbes, and further suppression of bone marrow cellularity did not occur when mice raised in germ-free conditions were treated with antibiotics. Our findings suggest that antibiotics suppress hematopoiesis not through direct toxic effects on progenitors, but by depleting intestinal microbiota.
Our study shows that the effects of antibiotics on hematopoiesis do not occur at a single developmental stage. Although primitive progenitors including HSCs and MPPs were depleted in the setting of antibiotic therapy, MPs remained present at normal levels. Therefore, an inadequate supply of primitive progenitors cannot explain the depletion of peripheral and bone marrow–resident terminally differentiated cells, including erythrocytes and granulocytes. Instead, our results suggest that the intestinal microbiota promote normal hematopoiesis at several stages: first by promoting activity of primitive progenitors and, second, by stimulating terminal granulocyte maturation. Because T cells are required for normal granulocyte maturation,22 a deficit in normal T-cell activation, as seen in antibiotic-treated mice, could slow granulocyte production. Therefore, we propose a model in which intestinal bacteria simulate basal interferon signaling, mediated by Stat1, that contributes both to MPP cycling as well as T-cell activation and granulocyte maturation (Figure 7C).
We found that the suppressive effects of antibiotics were phenocopied in Stat1-deficient mice. Antibiotic therapy has been implicated in suppressing Stat1 signaling in the gut,18 and our results suggest that these effects extend beyond the local intestinal environment.22 Stat1 is the major transcription factor downstream of pro-inflammatory interferon signaling, which activates T cells.23 Thus, our results suggest that basal interferon signaling is necessary to promote the progenitor cell cycling and differentiation needed to maintain normal hematopoiesis, as has previously been suggested.24 In contrast, we saw no contribution by MyD88-dependent Toll-like receptor or Nod2 signaling, two pathways known to affect microbiome and hematopoietic/immune system interactions.15,25
Cytotoxic CD8+ T cells and interferon signaling have been implicated in the pathogenesis of aplastic anemia,26-28 and in fact we find that antibiotic treatment led to CD8+ T-cell infiltration into bone marrow. However, the CD8+ T cells that we found were naïve, not activated, and we did not detect elevated levels of IFNγ in the bone marrow. These findings, combined with the observation that the bone marrow was suppressed in Stat1-deficient antibiotic-treated mice, argue against a cytotoxic role of CD8+ T cells in the bone marrow–suppressive effects of antibiotics.
Recent publications have illuminated the important contribution of multipotent progenitors to steady-state hematopoiesis.6,7 In our study, all types of multipotent progenitors were depleted without evidence of increased cell cycling, suggesting that adaptive proliferative responses were suppressed. In contrast we found that myeloid progenitors were maintained and had increased proliferation.19 These results contrast with 2 recent studies that showed decreased myeloid progenitors with antibiotic therapy15,16 ; however, those studies involved much longer antibiotic courses. Despite increased rates of MP division, granulocytes were diminished in the bone marrow, the major site of granulocyte reserve.19 The reduction in the granulocyte pool could be caused by increased utilization, tissue infiltration, or clearance of granulocytes, but we did not detect an increase in tissue granulocytes by gross or histologic examination in our mice, and a recent study found no evidence of increased clearance of granulocytes in the spleen during antibiotic therapy.16 Taken together, our studies are more suggestive of a maturation block in the final stages of granulocyte development that could be rescued by exogenous addition of G-CSF. These findings are consistent with a prior study demonstrating a requirement for T-lymphocyte activity in normal granulocyte maturation.22 The authors of that study speculated that CD4+ T-cell function may promote G-CSF production by endothelial cells. Our results demonstrating a depletion of granulocyte reserve in the marrow in the setting of diminished peripheral lymphocytes support this connection.
Our model of antibiotic-mediated bone marrow suppression mimicked important findings seen in antibiotic-treated patients.5 First, WBM counts and granulocyte reserve in bone marrow were significantly decreased, as has been described in patients with profound antibiotic-associated neutropenia. Second, the reversibility of antibiotic-mediated effects on hematopoiesis in our system is consistent with the observed recovery of cell counts upon withdrawal of antibiotic therapy in patients with antibiotic-associated bone marrow suppression. Despite these parallels, the mouse model differed from typical clinical findings in several ways. For example, the antibiotics most commonly implicated in bone marrow suppression in humans are the β-lactams.2,5 We tested single-agent β-lactam therapy in early experiments without any noticeable effect on the peripheral blood counts. Although we selected antibiotics and antibiotic concentrations that mimic human clinical conditions, these variables, physiologic differences between mice and humans, housing conditions, or other factors may have affected our results. It is also possible that our preliminary studies were simply underpowered. We report that antibiotic-treated mice develop lymphopenia, a finding that has not been associated with antibiotic therapy in humans. We speculate that the large changes in lymphocyte count may be a feature of mice housed in SPF conditions, which have pronounced peripheral lymphocyte predominance with smaller numbers of circulating/patrolling granulocytes compared with humans.
Our experiments using fecal transfer and germ-free mice indicate that depletion of the intestinal microbiome is critical in the development of antibiotic-associated bone marrow suppression. The interactions between commensal intestinal microbiota, gut epithelium, and immune cells in the intestine are complex.14,29,30 The enteral microbial communities of mice housed in the same cage are very similar,18 and we saw similar hematopoietic changes with antibiotic therapy in mice from both facilities in our study. However, we would expect patients to have more microbiome heterogeneity at the start of antibiotic therapy than laboratory mice and therefore more variability in changes of the intestinal microbial community with antibiotic therapy. This lack of uniformity may explain why bone marrow–suppressive effects during antibiotic use are highly variable between individuals or even within the same individual restarting the same antibiotic after count recovery.5
Other studies show that progenitors expand in response to enteral introduction of microbiota, in both germ-free and antibiotic-treated mice.15 Whether this response is related to repletion of microbe-derived metabolites or microbial antigen stimulation of hematopoietic progenitors remains to be elucidated. Further studies are also needed to assess whether supplementation with probiotics counteracts antibiotic-associated bone marrow suppression in patients on long-term antibiotics. Current evidence for the overall benefits of probiotic therapy while on antibiotics is mixed,31,32 and prevention of bone marrow suppression by use of probiotics has not been investigated. Assessment of the state of the patient microbiome at initiation of therapy may facilitate future studies to enable accurate prediction of which patients are at risk for antibiotic-associated bone marrow suppression.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. A. Matatall for invaluable assistance and support throughout this project; H. W. Virgin IV, M. L. Bettini, and R. Kellermeyer for useful discussions; S. Gottschalk, T. Horton, and V. Sheehan for sharing equipment; and C. Gillespie for editorial input on the manuscript. They are grateful to A. Swennes and S. Cormier at the BCM Center for Comparative Medicine’s Gnotobiotic Mouse Facility, as well as the Cytometry and Cell Sorting Cores at Baylor College of Medicine; and Texas Children’s Hospital with the assistance of Joel M. Sederstrom, Amos Gaikwad, and Tatiana Goltsova.
This project was supported by the National Institutes of Health (National Institute of Allergy and Infectious Diseases grant P30AI036211, National Cancer Institute grant P30CA125123, and National Center for Research Resources grant S10RR024574),the March of Dimes Basil O’Connor Starter Scholar Award, and the Aplastic Anemia & Myelodysplastic Syndrome International Foundation Liviya Anderson Award (K.Y.K.), and by National Institutes of Health, National Cancer Institute training grant 5T32CA009547, National Institutes of Health, National Institute of Allergy and Infectious Diseases grant 5U19AI109725-02, and the W. M. Keck Fellowship from Washington University (M.T.B.).
Authorship
Contribution: K.S.J., M.T.B., and K.Y.K. designed experiments, performed experiments, analyzed and interpreted results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katherine Y. King, Section of Pediatric Infectious Diseases, Department of Pediatrics, Baylor College of Medicine, 1102 Bates Ave, Suite 1150, Houston, TX 77030; e-mail: kyk@bcm.edu.